About Unicom
This innovation action will give a powerful impulse to implementation of ISO IDMP (ID of Medicinal Products) standards in EU Member States drug databases, supporting safe cross-border ePrescription/eDispensation and effective pharmacovigilance.
Once EU-interoperable data on medicines taken by patients become available, further benefits will accrue through better health data for improved clinical decision support, patient empowerment, public health and clinical research. New opportunities will arise for pharma industry, software developers, SMEs providing smart apps and others, thereby fostering their innovation capacity and competitiveness.
The many challenges still to be faced on this road will be tackled by a powerful consortium assembling all relevant actors, with critical mass for impact throughout the EU. After 10 years of development, the IDMP suite of standards is ready for implementation. Though some isolated implementation work has started, the time is now ripe for a more concerted effort towards large-scale implementation, contributing to this global interoperability endeavour and delivering benefits to EU citizens.
Project ambition centres on conversion of key regulatory and clinical processes to use IDMP. These information value chains must be converted over their full length from data input to data repositories to data usage.
Project work spans all three areas, focussing on the most challenging, the implementation of EU and national SPOR (substances, products, organisations, referentials) data bases, including establishing an EU Substance Reference System (EU-SRS). Such information is fundamental to cross-border ePrescription where safe dispensation may require reliable identification of substances in available products.
19 countries are represented, including 26 national Drug and eHealth Agencies. Stakeholders are involved through their associations. Duration is 4 years, budget € 21 m, with requested funding € 19 m.
Project Acronym:
UNICOM
Project Name:
Up-scaling the global univocal identification of medicines
Funding Scheme:
Horizon 2020 IA
Grant Agreement No 875299
Topic:
SC1-DTH-09-2019 – Scaling up the univocal Identification of Medicinal Products
Budget:
€ 20 730 965,50
EU contribution
€ 18 994 883,50
Duration:
December 2019 – November 2023
Information at your fingertips
Most resource-rich countries maintain at least basic national (electronic) repositories and data bases of medicinal products which have gone through the stipulated regulatory national process to be marketed in that national healthcare system.
Unfortunately, these uncoordinated national regulatory procedures have resulted in a host of unintended results and impacts endangering patient safety and hindering better healthcare service delivery, particularly in international contexts:
- Across health systems, the same medicinal product (MP) may have different names. UMC resource
Certain dosage strengths or package sizes may also vary or not be available. - Across countries, the same name may identify a different product with a different active substance.
- Across countries, the number and kind of medicines (MPs) authorised for national marketing differ very considerably (due to marketing strategies of producers, plus three different marketing authorisation procedures at EU and national levels).
- In cross-border ePrescription (eP) services this necessitates substitution in many, if not the majority of instances – if a specific MP is specified in a prescription.
Similar challenges apply to the electronic recording of medicinal products in other healthcare contexts, e.g. in electronic patient summaries, health records, clinical decision and ordering systems, or ePrescribing software.
UNICOM is dedicated to
- improving patient safety globally
- facilitating better healthcare for all
Patient safety issues, like medication errors, are due to numerous reasons, but many of them are related to a lack of readability of prescriptions, sometimes the partial absence of well detailed, structured and coded information on medicinal products – which also may somewhat differ depending on the national market, or missing information on other medicines taken by the patient.
The current digitalization of the healthcare sector may contribute substantially to reduce these threats, but without the adoption and concrete implementation of common conceptual and semantic standards to accurately identify and provide full, consistent and high quality data on all medicinal products available in the respective national or European market, the expected added value cannot be entirely delivered with data unable to travel between systems, regions, countries or use-cases.
All of this will be of great benefit to patients as well as healthcare professionals, and contribute to better healthcare for all across the Union.
UNICOM supports and enables the univocal identification of medicinal products by facilitating and accelerating the
- further development,
- implementation, and
- diffusion of ISO IDMP standards (IDentification of Medicinal Products)
across European health systems.
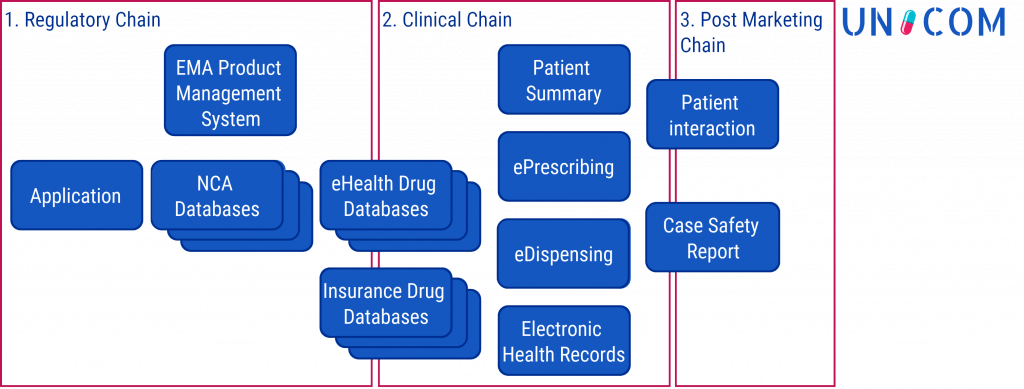
UNICOM supports
- regulatory processes of National Medicinal Products Authorities (NCAs) & the European Medicines Agency (EMA)
- global pharmacovigilance advancing cross-border digital health, particularly ePrescription services
- better healthcare, public health, medical research (e.g. Big Data analytics, AI applications)
Core objectives focus on:
- Adaptation and implementation of IDMP at EU and NCA level
- Adaptation of Member States cross-border digital health services (ePrescription; Patient Summary…) to IDMP
- Exploration and implementation of IDMP for pharmacovigilance reporting, Medicinal Product Dictionaries (MPDs), digital healthcare support services, patient empowerment.
The univocal identification of medicinal products is a global challenge. By going it alone, countries hinder the free flow of detailed, interoperable medicine information in the European Union, across the Atlantic, and globally. The UNICOM project joins forces with other stakeholders in our health systems to spearhead solving this challenge. It will accelerate the further diffusion of ISO IDMP standards (Identification of Medicinal Products).
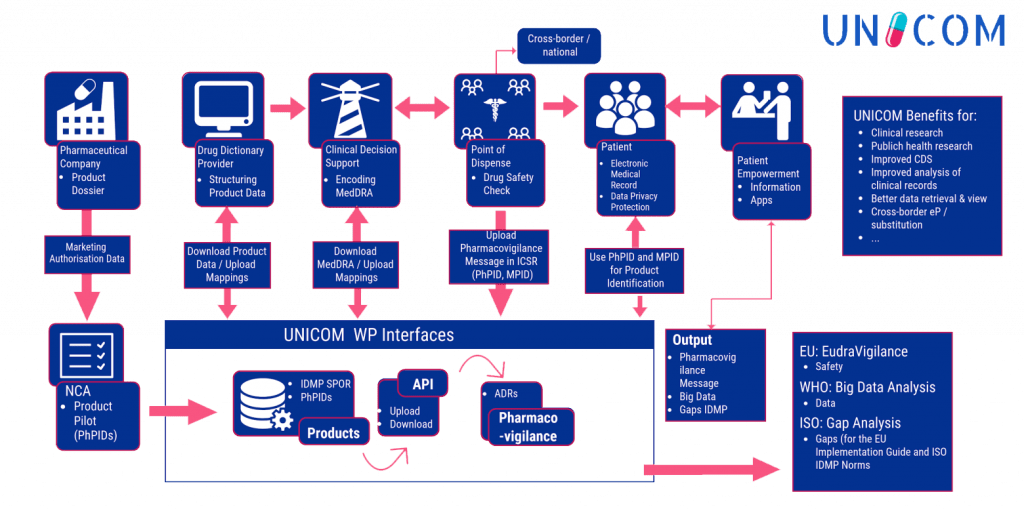
To achieve the objectives envisaged and reach the outcomes foreseen, UNICOM is organised along three closely interrelated vertical action lines:
- Implementation of IDMP at EU /National MPs data base level
- Adaptation of cross-border digital health services
- Exploration for pharmacovigilance services, Medicinal Product Dictionaries , healthcare services, patient empowerment, Big Data etc.
These three action lines are supported by two horizontal activity clusters:
- Further development of IDMP standards and implementation support
- Socio-economic impact assessment and sustainability strategies, scientific coordination, project management, awareness raising and dissemination.
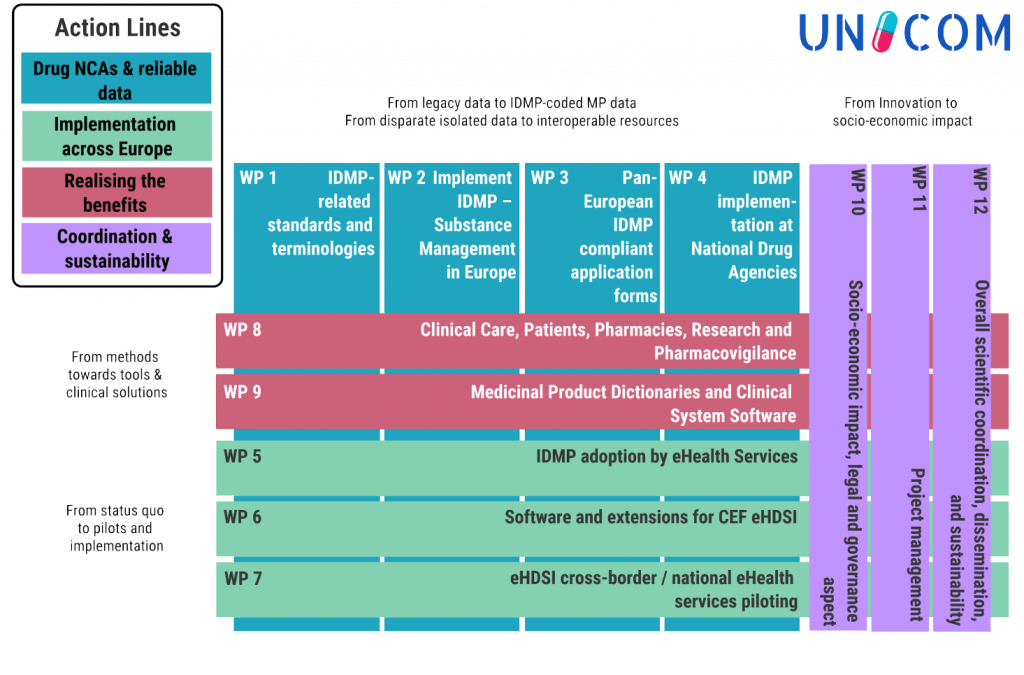
UNICOM will help to break down barriers hindering the free flow of detailed, semantically coded interoperable drug information across the globe, thereby
- facilitating data sharing amongst health professionals and patients everywhere
- providing interoperable information for all actors dealing with data and issues around medicinal products
This will deliver individual, social, societal, and economic benefits to
- Pharmaceutical companies applying for marketing authorisation of medicinal products
- National medicinal products authorities
- Providers of medicinal product dictionaries
- Clinical software producers
- Healthcare professionals (incl. pharmacists)
- Patients
- Start-ups developing intelligent apps for patient empowerment
- Cross-border digital health services (patient summaries, ePrescriptions, and beyond)
- Medical research
- Public Health
Core IDMP data value chain actors are consortium partners (26 National Drug and eHealth Authorities, Standard Development Organisations (SDOs), providers of cross-border ePrescription services, clinicians, patients, and many others).
Further stakeholders are involved through their associations or as experts (via an Advisory Board, expert workshops, meetings & conferences etc.)
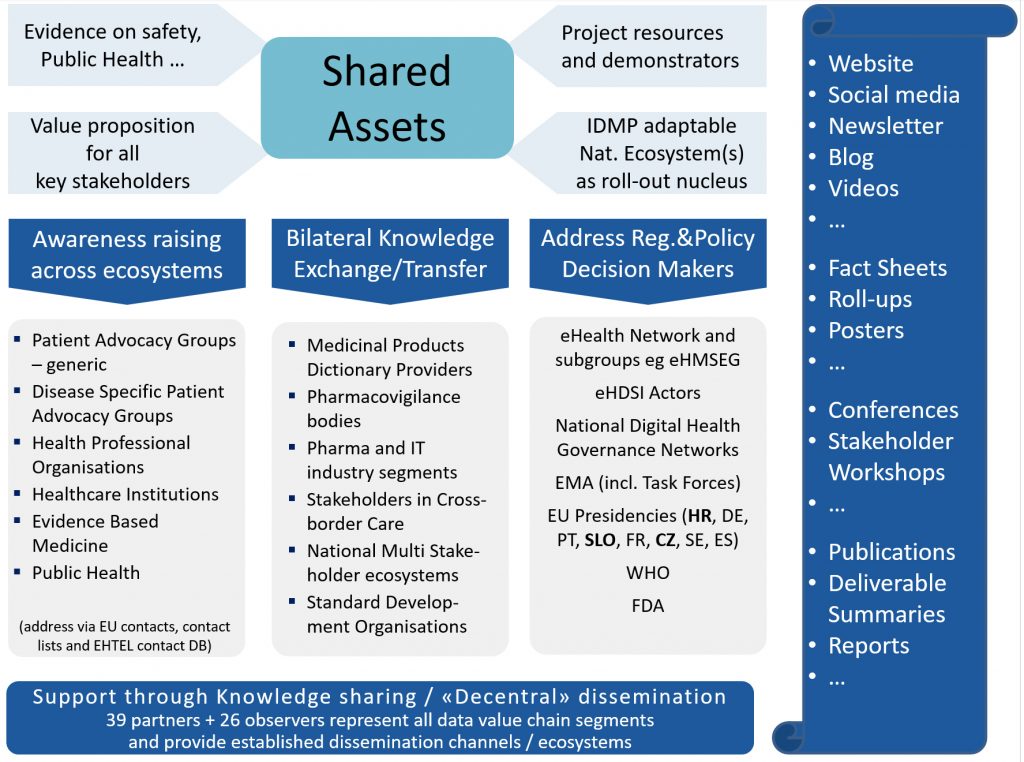
The impact and benefits for all stakeholders directly involved in the medicines data value chain, but also for health systems and societies will be multi-faceted and very substantial:
- Any patient can obtain seamlessly at least a medicine equivalent to the one prescribed in another country (in line with respective local regulations), and enabling patients is supported by simplifying product identification in patient-empowering systems.
- Clinicians reviewing a patient’s summary, electronic health record or other documents containing information on medications prescribed and dispensed understand fully the medicinal therapy information contained.
- An individual patient’s medication records originating from diverse sources can easily be integrated, validated, and used as input to computerised physician order entry (CPOE), clinical decision support and other (software) systems.
- Community pharmacists can fully identify what is the most appropriate medicinal product in their country that meets the specification and fulfils the therapeutic requirements of the product prescribed abroad, in accordance with their country’s laws and substitution rules.
- Different actors like regulators (e.g. EMA, national agencies); healthcare professionals, providers of national/regional medicinal product information systems; pharmaceutical companies; sponsors of clinical trials, medical researchers are enabled to meaningfully exchange MP data globally and share the same sources of information.
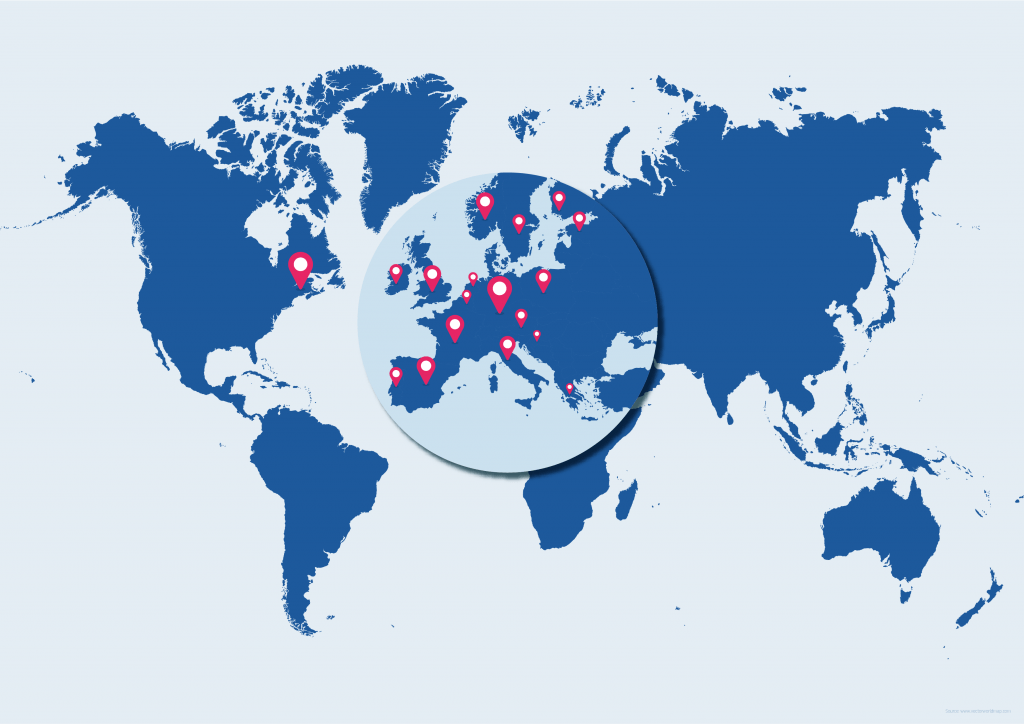
The project assembles consortium partners from
- 15 European Union member states
- The United Kingdom and Norway
- The United States of America
Through various consortium partners, UNICOM may also reach out to similar authorities and organisations on other continents once concrete results and lessons learned become available.
As part of its activities, UNICOM undertakes activities around IDMP to foster trans-Atlantic cooperation in the spirit of the “Memorandum of Understanding between The United States Department of Health and Human Services and the European Commission on Cooperation Surrounding Health Related Information and Communication Technologies”.
The eHealth, Well-Being and Ageing Unit (CNECT/H/03) of the Directorate-General for Communications Networks, Content and Technology (CNECT) of the European Commission (EC) in Luxembourg is supervising the project.
During its lifecycle, the project’s performance and results are evaluated regularly by an external team of reviewers appointed by the European Commission. The reviewers produce a report which includes global and specific recommendations on the overall management of the project, the different tasks performed, the quality of the deliverables produced and, as the case may be, on modifications of the remaining work.
UNICOM key deliverables are reviewed by carefully selected external experts, before being submitted to the Commission and, once approved, published on this website. This process is not a compulsory procedure, but UNICOM believes that, given the strategic importance of the project, it would bring additional value.
The project has also constituted an ethical board composed of well-known European legal and ethics experts who are not directly part of the project and can thus provide the needed advise in total independence.
Finally, the project foresees to establish an advisory board whose role will be to provide guidance on a number of issues once first results will be available.
Unicom will run for four years. Official start of this innovation action was 1 December 2019. The project is expected to end on 30 November 2023.
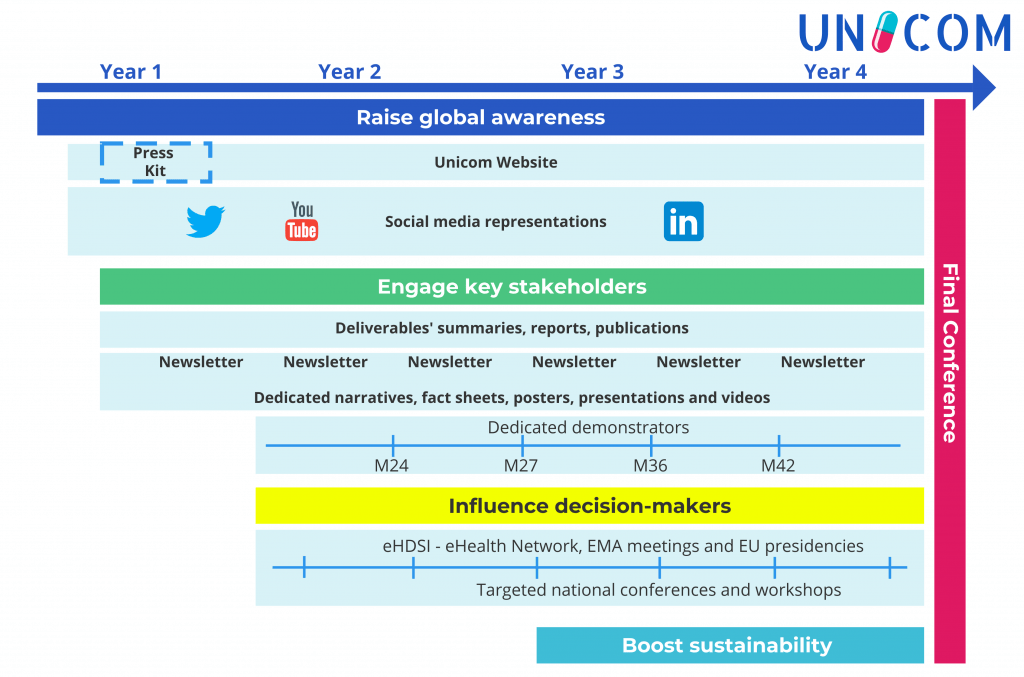
The official budget amounts to around € 21 million. European Commission (EC) funding is € 19 m; it is provided in the context of the European Horizon 2020 research and innovation programme under grant agreement No 875299. This support is gratefully acknowledged by all participants and stakeholders involved in UNICOM.
For several UNICOM consortium members, in particular selected National Medicinal Products Authorities, the funding received from the EC can be regarded as seed money instigating and often accompanying considerably larger investments in adapting or converting national medicinal products data bases, IT systems and regulatory processes towards IDMP standards. The same applies to UNICOM’s contribution to the creation of the European Union Substance Reference System (EU-SRS), which when finalised will provide input for the European Medicines Agency’s Substance Management System (SMS, a core element of the SPOR set of European medicines-related data bases ), a cornerstone for EU-wide implementation of IDMP.
To a lesser extent, the same holds for other beneficiaries of this grant. Furthermore, spill-overs to other European actors are expected. UNICOM is an excellent exemplar of European Union support initiating and fostering investments much larger than the initial funding.
