The Panhellenic Conference 2022 on finances and health policies is now over and UNICOM is very happy about the discussions, the results achieved and the next steps proposed.
The participants watched with particular interest the workshop organized by UNICOM partners, IDIKA and GNOMON Informatics, on Monday 12th from 14:30 – 18:30. The event brought together experts from the digital health ecosystem and pharma industry, to learn, network, and discuss the necessary feasible next steps to implement the necessary structural changes to implement the IDMP suite of standards about the accurate identification of medicines across all use cases. The workshop has succeeded to gather presenters- including Panagiotis Telonis (Scientific Administrator, Chief Information Office EMA)- who have been able to correlate the global picture with the Greek reality.
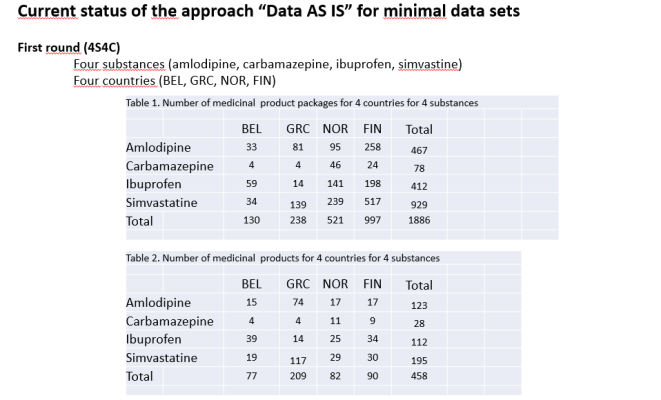
Alexander Berler made a presentation of the UNICOM project and its strategic contribution to the making of a European Health Data Space, while Robert Vander Stichele from I~HD provided very inspiring examples of the benefits of a wide ISO IDMP implementation with a detailed analysis of an harmonisation between the products marketed related to 4 different substances in 4 different countries. Ioannis Asproloupos from IDIKA presented the latest developments of myHealth@EU and the preparations made for wave 6 in IDIKA. Dr Dimitris Kounalakis presented the Master Values Catalogue (MVC) used in cross-border scenarios while Mrs K. Chronaki from HL7 presented the patient leaftletuse case (electronic Product Information – ePI). Dr Haralampos Karanikas and Argiris Gkogkidis presented the new tools and components that are being developed in UNICOM to support Member States in their cross-border services. Finally, Dimitris Katehakis presented the relationship of the National List of Medicinal Substances with the IDMP encoding.
The workshop was very well received by the Greek Medicinal Products Community: UNICOM will also distribute in the coming days a didactic leaflet translated in the Greek language which should support a dissemination to an extended Greek audience.
UNICOM also used this opportunity to organise a closed executive working meeting on Tuesday 13th with relevant Greek stakeholders, the Gnomon team and Robert Vander Stichele from I~HD to discuss pending issues and make progress related to the work on the production of the Greek pilot pharmaceutical data for the four substances. The comments on the Greek data were scrutinized. After checking the latest version of the Greek data, revised by IDIKA with the support of the Gnomon team, almost all major issues were solved. Only a few remaining issues needed some complementary actions due to be solved under short notice (i.e. URL of eSPC and Patient Leaflet, Short name of company etc.) According to Robert Vander Stichele, after adding the SPOR codes and the short company names, the Greek data will have reached the highest level of quality best of this minimal data set.
The public session on Wednesday 14th together with Gravitate Health was also very well attended and has been preparing the ground for a reinforced collaboration between the two projects.
We are excited to move forward with the testing and look forward to the results. UNICOM wants to thank the Greek agencies, GNOMON and I~HD for their concrete involvement and engagement during those three days.