The fifth trans-Atlantic workshop orgnanised in february 2023 was attended by representatives from UNICOM, US and EU regulators, biopharmaceutical manufacturers and technology vendors. Meeting discussions focused on global IDMP Implementation initiatives, with presentations provided by regulators (FDA, EMA, MEB), biopharmaceutical (Bayer, Roche and Sanofi) and WHO-UMC and representatives.
A review of the organisations involved in various aspects of IDMP implementation (e.g., regulator and industry-focused, data standards, education and training, etc.) reveal no redundancy in work efforts and key activities complement one another, which promotes a positive, synergistic message and approach towards consistent global IDMP implementation and refinement of the underlying IDMP standards open for ISO systematic review.
The GIDWG (Global IDMP Working Group) is a key stakeholder group comprised of global regulators and industry participants. The GIDWG was established to focus on operational aspects to identify and develop consensus processes and best practices for global PhPID creation and maintenance. The WHO Uppsala Monitoring Centre (WHO-UMC) (a UNICOM affiliated organisation) is working to develop and test solutions to generate and maintain global PHPIDs.
Larry Callahan (FDA) provided an update on G-SRS, focusing on high-level statistics about the state of the system’s database/dataset, G-SRS data pipeline and software development roadmap. Current issues pertaining to manual data entry (due to PDF submissions) and restructuring of incoming data to conform to new data exchange formats was presented. Information about ongoing development to facilitate submission of structured information earlier in the drug development lifecycle (e.g., new in-vitro pharm module) was presented and closed with a discussion about the formation of a new governance consortium focused on strategic goals to promote G-SRS as a truly global platform and improve the quality and availability of harmonized substance and product information around the world. Ta-Jen Chen summarized the release of FDA’s IDMP implementation guidance and mapping of other IDMP use cases and FHIR-related development, such as mapping between HL7 SPL FHIR and PQCMC.
Malin Fladvad of WHO UMC (a UNICOM partner organisation), provided a more in-depth overview of GIDWG activities based upon a phased approach. A summary of each phase, including completed work was given. PhPID generation and maintenance based upon substance IDs, dose form, and strength was discussed, including snapshots of the proposed workflow, request and validation processes. Phase 3 activities are underway, focusing on process documentation. In the future, a fourth phase of work will be proposed to focus on developing additional business rules and investigating process automation. Relevant lessons learned pertaining to ID creation, especially related to substance reference strength and dose form characteristics will be considered among other requirements during the ISO IDMP standards systematic review process.
Isabel Chicharo and Panagiotis Telonis of EMA provided updates on the current status of EMA’s IDMP Implementation, with a focus on EU compatibility and compliance. EMA’s SPOR integration was highlighted and how data is stored and used in agency systems, domains, and processes throughout the regulatory cycle (e.g., research, development, registration, and postmarket surveillance). A focus for next year will be on EU-SRS use and integration and the need to scale information globally. Additional activities include ongoing collaborations with FDA to develop and test HL7 FHIR to support other types of regulatory submissions, such as PMS, UPD, ePI, DADI, PQ/CMC and transition of HL7 SPL.
Joris Kampmeijer discussed IDMP implementation, focusing on some of the downstream benefit(s) to healthcare, such as cross-border prescriptions. A discussion of industry perspectives on IDMP implementation was moderated by Vada Perkins, with presentations from Sheila Elz (Bayer), Rodrigo Palacios (Roche) and Elisabeth Godet (Sanofi). The need for a global IDMP terminology and ID maintenance organization for IDMP terminology and identification was discussed because multiple regional stakeholders create their own standards and IDs. The GIDWG could potentially serve as a bridge to help address operational gaps among regulatory stakeholders processes, and systems. Sheila Elz (Bayer) presented an overview of the ISO IDMP Logical Model and the IDMP Ontology work items. A goal of the IDMP Ontology project is to define requirements for a consistent approach and tools due to diverging IDMP implementations, which create more siloes and increased cost of point-to-point integrations and risks to patient safety. IDMP Ontology supports a universal implementation of IDMP, utilizing a collaborative implementation to create interoperability by design, while also ensuring that individual use cases and data challenges are addressed. Another benefit of the IDMP Ontology is the ability to speed-up digital transformation and IDMP implementation across functions and jurisdictions. Elisabeth Godet (Sanofi) provided perspectives on IDMP implementation, focusing on the EMA vision of the product lifecycle management and moving to a data-centric model. Key focus areas include EU digital transformation, internal testing, supporting “go-live” of EMA’s PLM Portal, implementing data centric processes globally, and providing SME input on the product lifecycle. Rodrigo Palacios (Roche) provided an overview of how IDMP is being leveraged to support compliance and digitalization activities. The current state for many biopharmaceutical companies is that product information is inconsistent, siloed and duplicated across the enterprise, which requires significant resources (time, people, financial) to extract, transcribe and reformat data to achieve IDMP-compliancy. The same data is often repurposed for other downstream regulatory processes (e.g., product registration and listing, Pharmacovigilance), which limits timely access to high quality data in critical business functions. Companies need solutions to speed up transition from siloed, paper-based frameworks to more agile, enterprise-level and datadriven submissions framework leveraging common Master Data Management (MDM) principles.
Christian Hay summarised the ISO Systematic Review (SR) process, which requires that standards be assessed for updates to better align with real-world implementations and/or changes in any underlying aspect (e.g., science, technology, policy) that may impact global adoption and use of standards. Specifically, several IDMP standards will be subject to SRs beginning late 2023, including other IDMP related standards such as DTS 6476 Logical Model, DTS 5499 Therapeutic indications, Revision TR 14872 Core principles for maintenance of identifiers and terms, new IS for IDMP Core vocabulary (terms and definitions) for the IDMP Standards and new standard for defining a Methodology and Framework for the Development and Representation of IDMP Ontology.
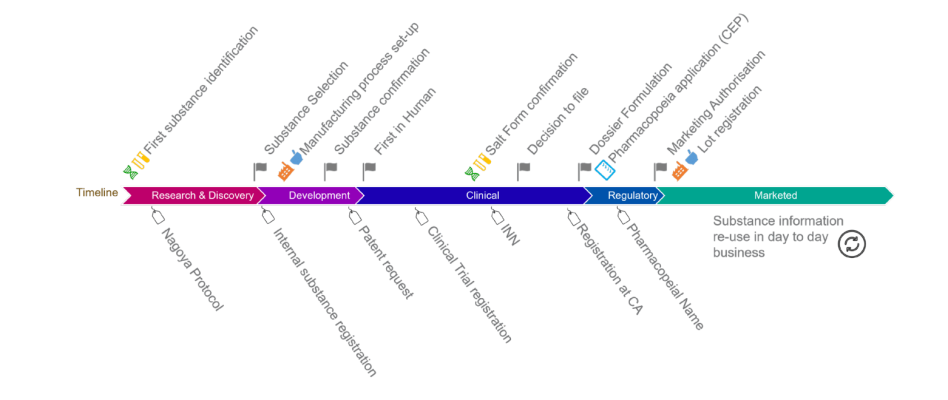
Three IDMP breakout sessions focused on different phases within the drug development lifecycle and prioritized use cases (e.g., drug shortages, pharmacovigilance and cross-border prescription) where IDMP identifiers could be generated and used internally (e.g., as master terms and IDs used in regulatory submissions) and externally (as harmonized terms and IDs that can be used to map and/or reconcile IDs used in other regions and healthcare applications.) Other use cases where IDMP information could be leveraged was discussed, such as ongoing FDA work to further structuring of product quality and Chemistry, Manufacturing and Controls information (PQ/CMC), Knowledge-Aided Assessment and Structured Applications (KASA) and improved integration and knowledge sharing between clinical trial and marketing authorisation data. User profiles (personas) were also discussed, based upon how different users could leverage global PhPIDs to support regional coding issues, language translations and other pharmacy applications.