This year’s ePI Summit was hosted by ROTE LISTE®. In an era where innovation is transforming every industry, the pharmaceutical sector is no exception. This year’s summit has explored the latest developments, opportunities, and challenges in electronic product information.
Key experts from industry and authorities will share their expertise on various topics including:
– The EMA-HMA-EC ePI pilot
– The influence of AI on pharmaceutical product information
– The status of requirements and impact of IDMP on ePI
– The impact of the EU pharma package on ePI
ePI is authorised, statutory product information for human medicines (i.e. summary of product characteristics, package leaflet and labelling) in a semi-structured format created using the EU ePI Common Standard. ePI is adapted for electronic handling and allows dissemination via the web, e-
platforms and print.
Agreement of a common standard will avoid a situation where multiple different standards are developed and used in different parts of the EU, which would generate unnecessary complexity, impede access to information and require multiple interfaces between standards, restricting flow of data
On Day1, UNICOM was present and complemented a presentation made by EMA. EMA has been conducting a pilot since July 2023 that will end in July 2024. The first ePIs have been published.
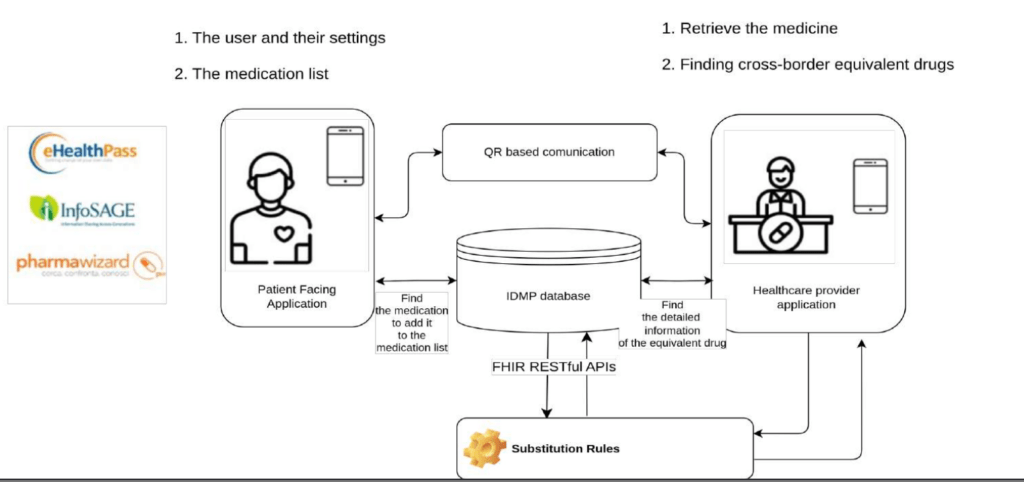
Marcello Melgara explained how UNICOM has created a move towards a global semantic interoperability for Medicinal Products which will facilitate data sharing across the full life cycle and will empower all actors involved in handling MP information: it is providing thus a solid fundation for ePI as alreday demonstrated by UNICOM collaboration with the IMI project Gravitate Health.
UNICOM has created the first ever IDMP compliant pilot database which was demonstrated in several Patient Facing Apps: