UNICOM hosted in March 2024 a workshop that aimed to engage stakeholders in discussions about the advantages,challenges, obstacles, and timelines related to the implementation and utilisation of IDMP in the clinical realm. This exclusive, one-day event, accessible by invitation only, was designed to showcase
the advancements achieved within the UNICOM project and underscore the ongoing efforts to unlock
the benefits of IDMP.
Invited stakeholders included:
- National Competent Authorities
- eHealth national organisations
- Medicinal Product Dictionaries providers
- EHR vendors
- Pharmacovigilance specialists
- SDO representatives (ePrescription, SNOMED CT, etc…)
- Hospital Pharmacists
- University representatives (MD)
The workshop served as an opportunity to involve participants who were not all actively part of the
UNICOM project discussions.
IDMP is increasingly referred to in the clinical domain as exemplified by this joint statement of European Academy of Paediatrics and European Confederation of Primary Care Paediatricians: “Over 85% of all European children are vaccinated and monitored by the WHO. The WHO classification system Anatomical Therapeutic Chemical (ATC) registers vaccines and medicines. A more detailed International Organization for Standardization (ISO) suite of IDMP (Identification of Medicinal Products) standards is coming. These standards provide an international framework to uniquely identify and describe medicinal products with consistent documentation and terminologies and to ensure the exchange of product information”.
Three illustrative use cases were explored in-depth during this meeting: e-prescription, adverse events reporting and drugs shortage.
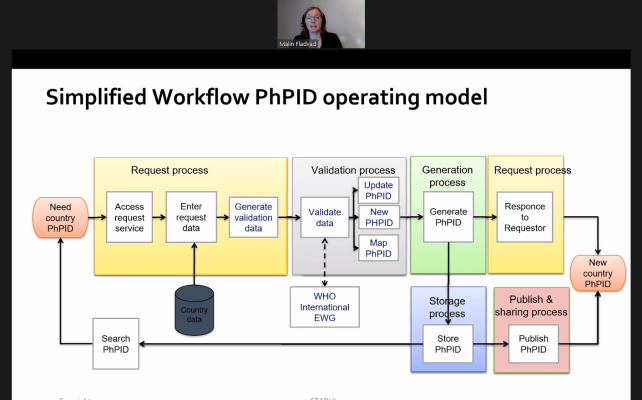
There was a consensus that the most important objective is to dispose of a global IDMP implementation, which implies availability of global substance identification and global pharmaceutical product identification. Awareness was also raised about the “portfolio of identifiers” which might be part of MPDs to enable linkage of products from packaging level to PhPID level, and vice-versa.