The authors of the article describe the challenges and learnings in the process of refactoring internal system for tracking the records of marketing authorization procedures and medicinal product database in the Agency for Medicinal Products and Medical Devices (HALMED) in order to comply with ISO IDMP (International Organization for Standardization, Identification of Medicinal Products) standards.
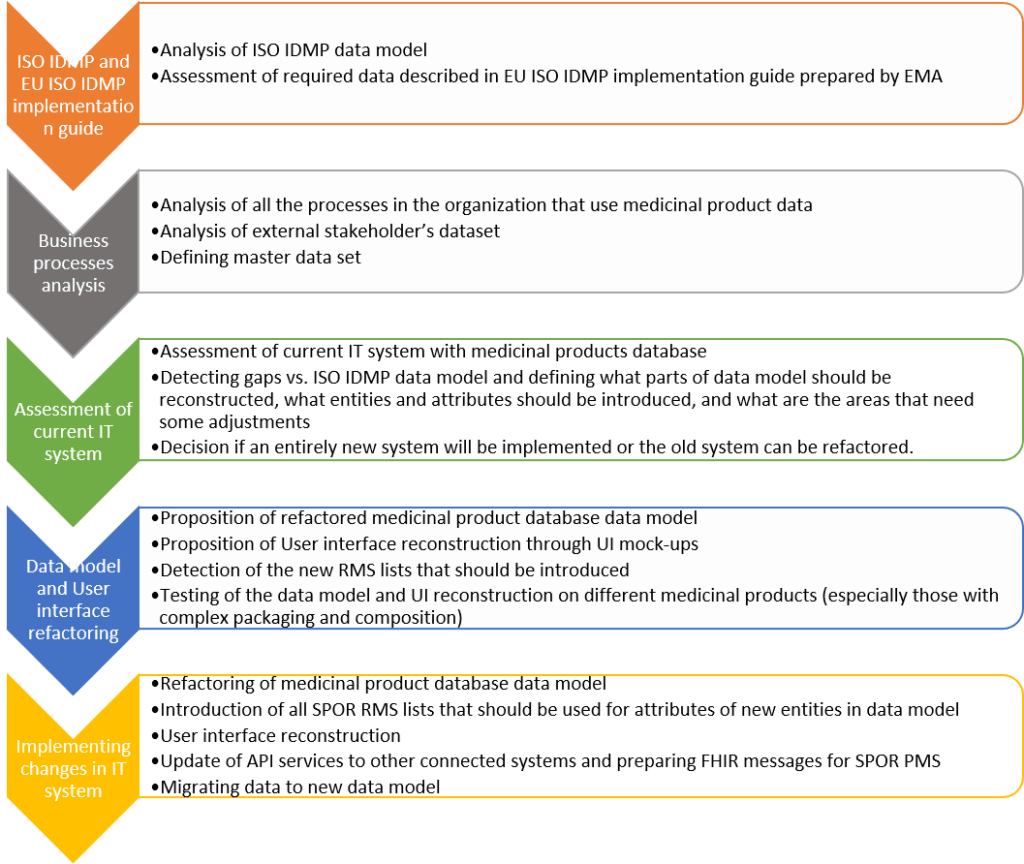
The implementation process is very demanding for the industry, the healthcare organisations or the regulators. It requires a deep assessment of all internal business processes that rely on medicinal product data and an analysis of external stakeholder’s dataset needs so that a master data set can be defined; Organisations need to become acquainted with the ISO IDMP data models and the EU IDMP Implementation guide. One of the first decisions to be made is whether an entirely new system (off-the-shelf or custom made) needs to be implemented or whether the old system can be refactored. HALMED opted for the refactoring of the existing system.
HALMED’s plans include the medicinal products data model reconstruction, user interface adaptation, changes on the synchronization processes and establishing connection with SPOR (Substances, Products, Organisation and Referentials) data management services. In a response to the introduction of the ISO IDMP set of standards, the EMA (European Medicines Agency) initiated the SPOR data management service project, with the objective to provide the framework for standardization and structured data in medicinal products description.
The article will be presented to the public during the eTELEMED 2022 conference on Thursday, June 30th, from 15:45 – 17:45 by Maja Fatiga