The Digital Application Dataset Integration (DADI) Network project will replace PDF electronic application forms (eAF) used for regulatory submissions with web-forms, making the future form-filling and submission-handling process more efficient.
This is the first of the three forms to be introduced. The form for initial market authorisation will be introduced at a later stage with no deadline set for now.
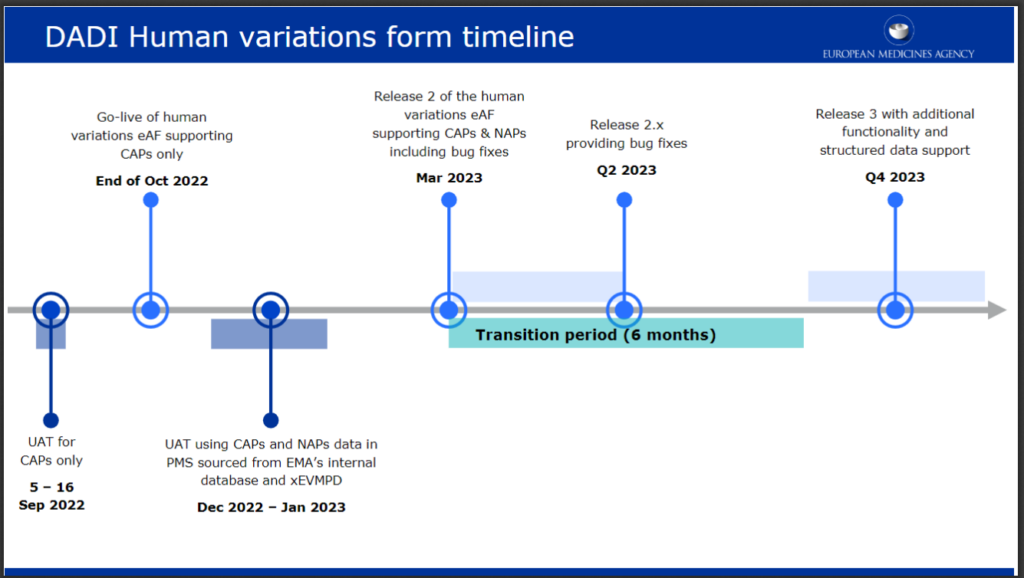
From now till end of October, a number of events in order to bring as many stakeholders on board has been planned.
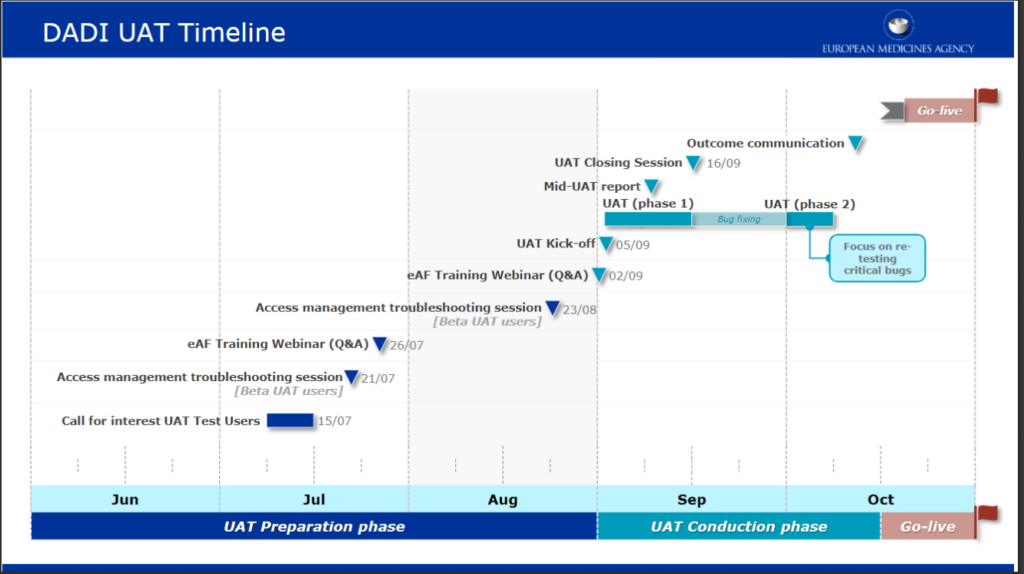
A first training session has been organised by UNICOM on July 15: It targeted all European Medicinal Agencies Chief Information officer and chief architects.
The recording of the training is available here:
To encourage all stakeholders to register on the portal to effectively support them ahead of the go-live date, EMA is now organising two supplementary public eAF training and Q&A webinars on July 26 and on September 2.
These events are training webinars for industry and national competent authorities’ stakeholders wishing to learn more about access management aspects related to the new eAF dedicated portal and the procedure to fill in a web-based eAF at go-live.
During the sessions, a demonstration focused on access management and the User Interface will be performed in order to showcase how to register and access the Portal, how to fill in the web-based eAF, select products and export the forms. We encourage live participation but those sessions will also be recorded and be made available.
You are most welcome to share your questions ahead of the webinars by submitting them at
Furhermore, EMA has also published the eAF Portal guide to registration: it aims to support the users of the eAF Portal in completing the registration steps before accessing the platform. Most of these steps are independent from the eAF Portal and correspond to those to obtain registration to use other European Medicines Agency (EMA) systems, such as Management Services for Substances, Products, Organisation and Referentials (SPOR).
End of October is only the first of a series of milestones with a first focus on Centrally Authorised Products (CAPs). From December 2022 to January 2023 the UAT will be extended Nationally Authorised Products (NAPs) and new releases aimed at fixing identified bugs will be produced end of Q1 and Q2 2023. The release 3 foreseen for Q4 2023 will provide a new breakthrough as it will support to structured data and will propose new critical functionalities. The level of structuration of data will also increase over time in full compliance with the SPOR developments.