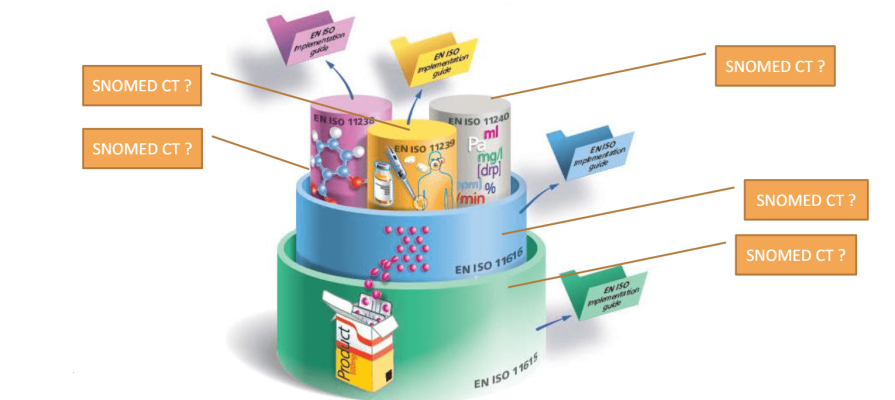
The next UNICOM Community of Expertise is scheduled on Friday 28 May 2021 between 3:00 – 4:30 PM CEST and will focus on the representation of clinical information related to medication using SNOMED CT and other standards
IDMP concrete implementation also requires semantic interoperability and use of adequate terminologies.
This webinar will illustrate the use of SNOMED CT and other standards for the representation and use of clinical information in a selected number of scenarios across the UNICOM landscape of IDMP implementation. The MHRA (UK) will demonstrate the use of SNOMED CT coded data in an actual electronic health record system to populate an individual case safety report (ICSR) in case of an adverse reaction to medication. MedDRA and EDQM Standard Terms are the terminologies of choice for the ICSR. The objective of the webinar is to identify and better understand the need for coordination across standards with respect to the representation of clinical information. This includes the use of mappings between different terminologies and value sets as required in different domains and the efforts needed to maintain such mappings.
Date: Friday 28 May 2021
Time: 3:00 – 4:30 PM CEST
Presenters:
Jane Millar (SNOMED International, WP 1)
Ian Green (SNOMED International)
Sarah El Amin (MHRA – UK Medicines and Healthcare products Regulatory Agency (MHRA)
Panelists:
Toni Morrison (SNOMED International)
Barry Hammond (MedDRA, WP 1)
Chris Jarvis (EDQM, WP 1)
Kate Bateman (MHRA)
Moderators:
Christian Hay (GS1 and ISO TC 215/WG6, WP 1)
Robert Stegwee (CEN TC 251 – WP 1)
Link: You can already register with the following link:
https://us02web.zoom.us/webinar/register/WN_qymogaTSSq6tPIGKu9clcA