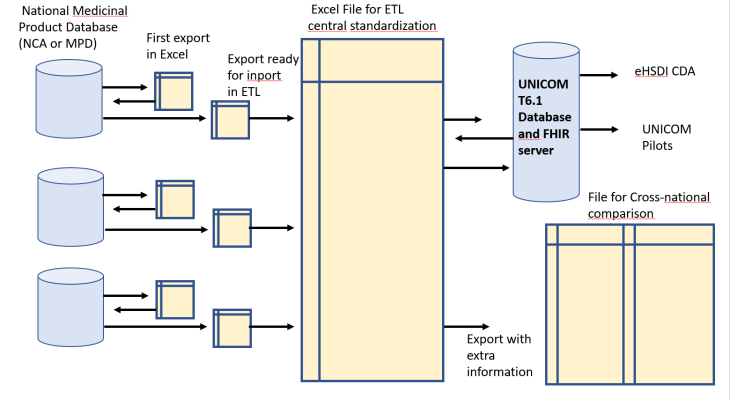
The process consists in collecting Data “AS IS”, and doing a central standardization to EDQM, limited to the minimal attribute list (variables relevant for pilots) and limited to 4 single and simple substances – (amlodipine, carbamazepine, ibuprofen, simvastatine) but requiring all products for these 4 substances. This exercice has been made in six countries: Norway, Belgium, Greece, Italy, and now also for the USA and Finland. Data originate from NCAs or National Medicinal Product Dictionaries (with approval of the NCA) with minimal effort required from the source.
The ambition is to initiate a documented, structured ETL process with centralized standardization, in constant feedback with the sending organisation. Data are subject to the validation processes of UNICOM (conformity to the IDMP model and FIHR specifications). The validated database is instrumental to the pilots of UNICOM (eHSDI cross-border services, substitution component, cross-national comparison, linking to international pharmacotherapeutic classifications, patient-facing apps, experiments in PhPID production)
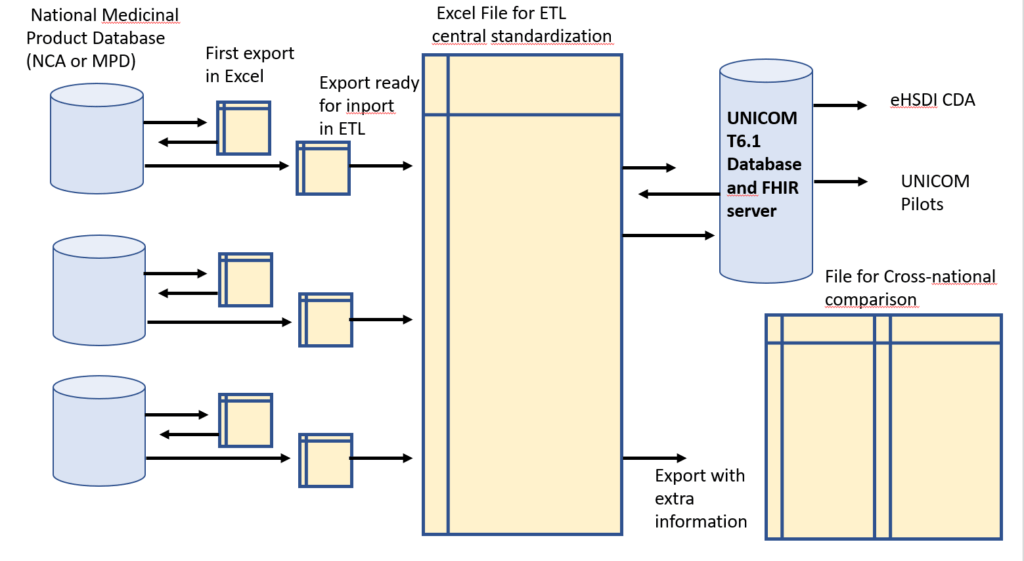
For each of the six countries , the following steps are followed for the data export and the ETL process
1.Agreement between sending organisation (NCA/MDP) and UNICOM for sending data “AS IS” on the 35 substances of the UNICOM Pilot Product List.
2.First analysis of the data “AS IS”, identifying the gaps in information needed for the ETL Process
3.Feedback iteration with the sending organisation and further completion of the data
4.Initiation of central standardization of the national data set (manual effort, hence limited to 4 substances)
5.Integration with data sets of other countries with consistency check: dose form of originators, strength expression, specifiers (only for amlodipine and ibuprofen) and orthography (capitals, trailing “e”s)
6.Import procedure into T6.1, with programmed checks) and check against FHIR specifications
7.Feedback with the national organisation, as a learning experience for the other substances on the Pilot Products List.
8.Release of the national minimal data set for the pilots.
Aside from the database itself, this exercice has thus also triggered intensive and instructive interactions between the UNICOM experts and the involved NCAs contributing to support the creation of a close and structural between the regulatory and the eHealth domain.
______________________