The implementation the ISO standards for identification of medicinal product (IDMP) is a key effort for global pharmacovigilance. One of the cornerstones of improving identification and analysis of safety issues is the Pharmaceutical product Identifier (PhPID) which has the potential to associate medicinal products of similar composition in different countries by representing information about substance, dose form and strength at different levels of precision.
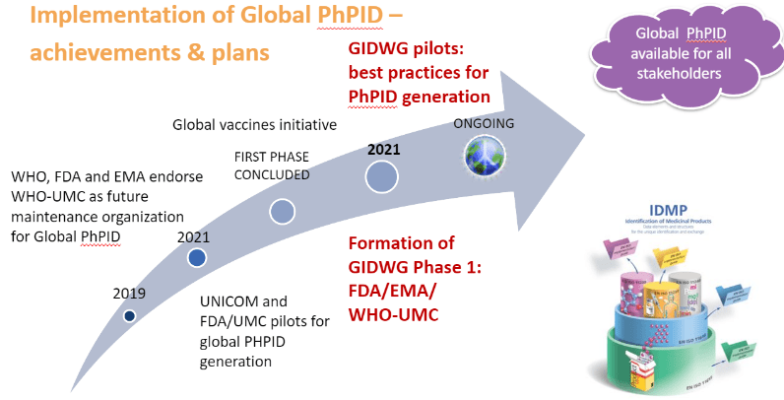
Recent progress in global PhPID implementation was initiated already in 2019, when WHO hosted an IDMP workshop with representatives from several regulatory authorities and industry. The outcome from the workshop included proposing WHO-UMC as the maintenance organization for global PhPID and a plan to further explore use cases, processes and best practices, including pilots, for PhPID generation. Shortly after, the European UNICOM project for IDMP implementation started and with the roll-out of Covid-19 vaccines globally, the need for global harmonization of medicinal product information and global PhPIDs became even more evident.
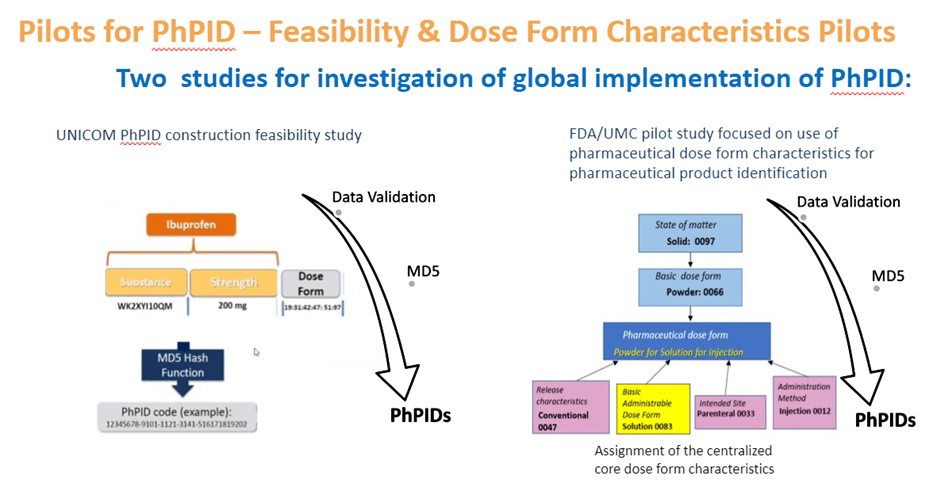
Two major pilots for PhPID have been completed so far. The UNICOM feasibility pilot was performed during the fall of 2020 and reported successful PhPID generation based on the concepts described in ISO IDMP for substance, dose form and strength and their numerical representations.
An additional larger pilot was performed by US-FDA and UMC during the spring of 2021, with the focus on challenges regarding dose form and mapping to regional terminologies. A centrally maintained set of dose form characteristics for use in global IDMP and generation of PhPID was proposed as a viable solution for dose form expression. In addition, several issues for unique identification of substance and expression of strength were also identified, resulting in proposed actions for updates of the ISO IDMP standards and continued work on best practices and business rules.
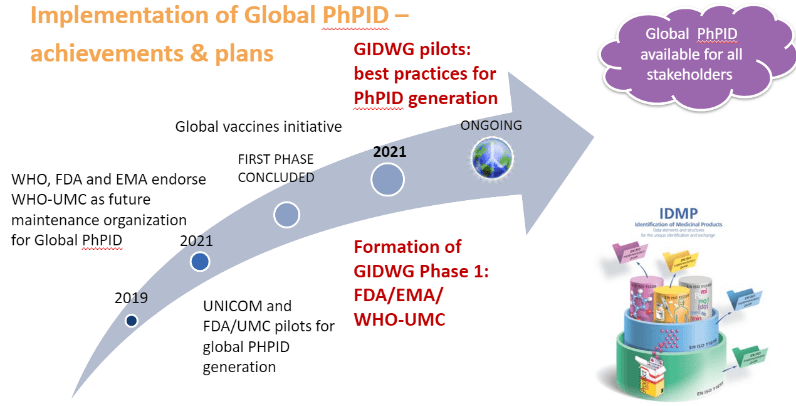
To continue to explore the PhPID on a global level, a Global IDMP working group (GIDWG) was formed in Oct 2021 with the goal to pursue projects leading to the establishment of a framework for the global implementation of the ISO IDMP standards and maintenance of global identifiers. The first phase of the working group will have members from EMA, FDA and WHO-UMC but the goal is to expand to a larger group as IDMP implementation efforts accelerate.
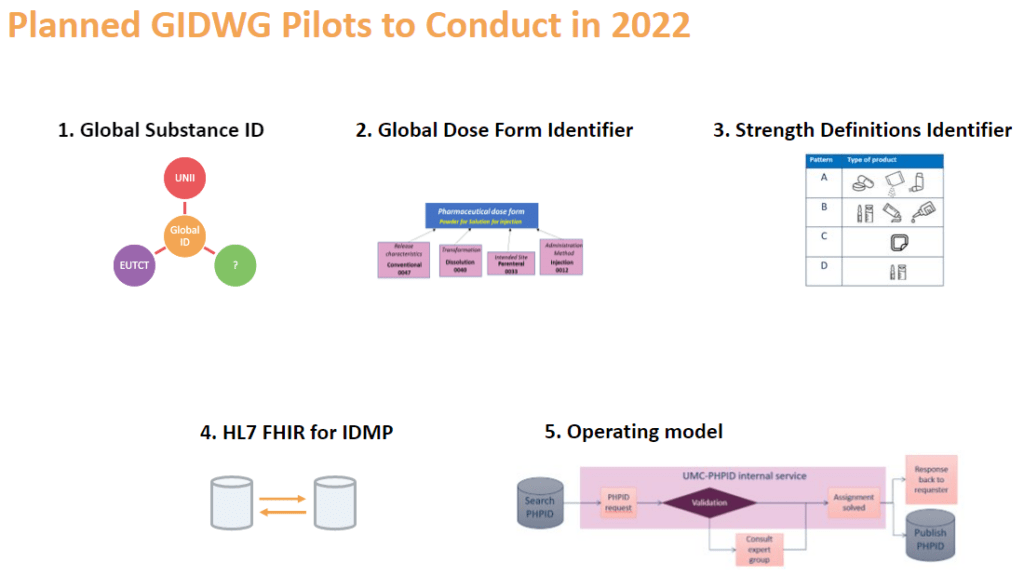
For 2022, GIDWG is planning for five additional projects to continue to work on the different elements for PHPID production; Global Substance ID, Global Dose Form Identifier, strength definition identifier, HL7 FHIR for IDMP and a Global PhPID Operating model