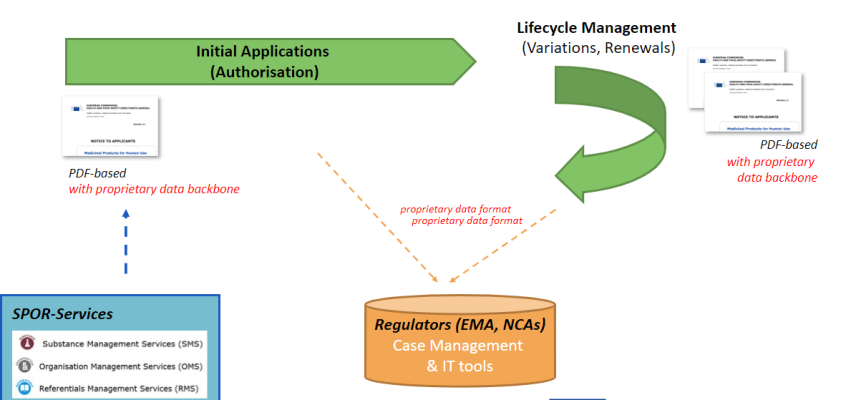
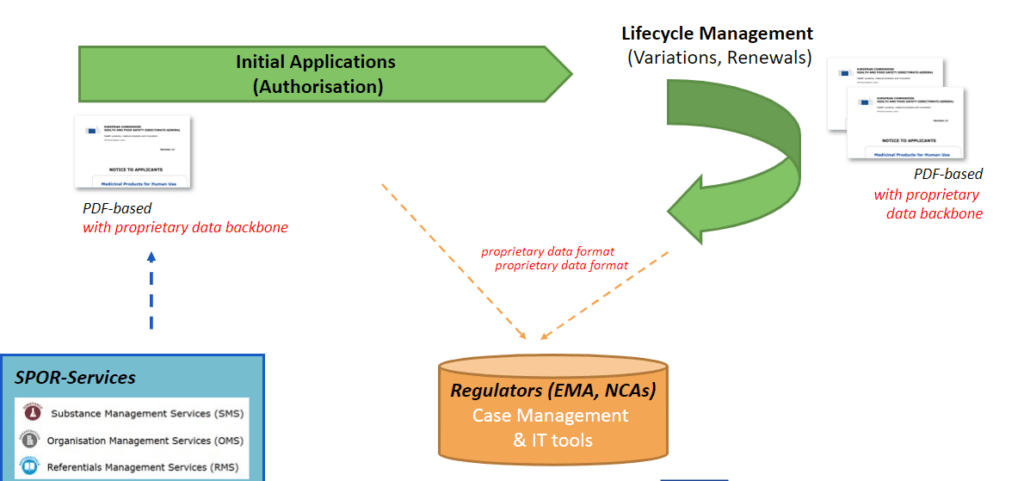
At the moment neither application forms nor the tools for initial authorisations, variations and renewals of medicinal products are compliant to the IDMP standards. It is thus not yet currently possible to start, automate and feed regulatory processes with IDMP compliant/structured data and easily re-use the data in EU-wide eHealth services. But this will incrementally change in 2022!
Applying for authorisations for medicinal products and managing their life cycles is a regulated process supported by electronic application forms and supporting electronic tools. UNICOM WP3 objective is to adapt the application forms and required tools towards the IDMP standards and to increase the usage of EMA’s SPOR. It will therefore deliver web-based application forms compatible with IDMP standards
Differences between the current application data format and IDMP have been identified and discussed with regulators and impacted stakeholders. UNICOM focus is on the human domain but synergies with the veterinary domain may also be realized.

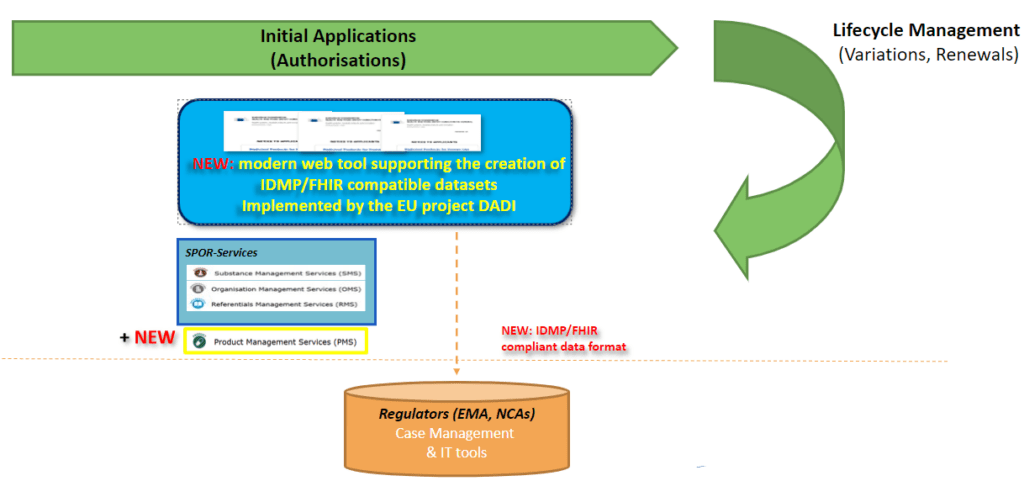
A decision was taken in 2020 to align the technical framework for the new online application form tool to the EMA technology strategy and for UNICOM and EMA to work together within the “DADI” project to replace the current PDF-forms; while the new tool will be implemented in EMA’s technical platform (Microsoft PowerApps) organised and executed by EMA, UNICOM WP3 keeps on contributing e.g. by acting as the product owner (jointly with a second product owner from EMA).
A permanent requirements group co-led by the product owners was then established: this group meets on a (bi)-weekly basis and is in close relation with the electronic Application Forms Maintenance Group (eAF MG).
Significant progress has been made in 2021 in the work on variation form.
The functionalities supported by the DADI User interface for variation forms is currently undergoing advanced testing. The utilisation of PMS (product Management Service) data will follow in the next testing iteration.
“ Experts from the UNICOM consortium and the EMA are working together intensively and extremely well on this innovation. There are regular alignments of features and demos of development results with users (regulators and companies). These are the success factors of the agile implementation concept. In 2021, a milestone was reached with the publication of the first test version of the new application tool for variation forms.” said Georg Neuwirther, Lead UNICOM WP 3, and Head of IT Austrian Medicines and Medical Devices Agency .
EMA has thus decided that it was now time to inform and involve deeply the Industry and the National Competent Authorities with two webinars planned for early 2022.
- The first webinar on the 18TH of January will be focusing on DADI roadmap & objectives for both centrally and nationally authorised products (CAPS & NAPS), DADI’s connection to EMA’s Regulatory Business Optimisation and will explain the main changes introduced by DADI and its impacts for the pharmaceutical industry. Registration is now closed but organizers are considering a broadcast on EMA youtube channel. People are warmly invited to .">submit their questions in advance.
- The second webinar on the 25th of January is targeted at technical experts from industry and national competent authorities wishing to learn more about the design of the FHIR message for the first form to be updated: Human Variations. You may register here.
DADI has also produced a useful document gathering all the main questions and answers.
UNICOM considers that the incumbent DADI roll out will be a major milestone on the road to a harmonised description and identification of medicinal products in Europe.