Our next Community of Expertise will have as focus: “Semantic specifications for eHealth services“
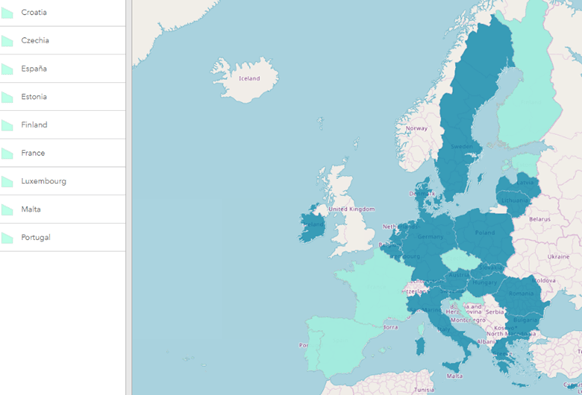
At the end of 2021, 9 European countries had established an operational national contact point for eHealth. 4 countries (Finland, Estonia, Croatia and Portugal) had activated ePrescriptions services while 5 other countries (Cyprus, Greece, Sweden, Ireland and Poland) are now very near to join the club of early adopters. There have been some 23 330 eDispensation performed since early 2019, mainly between Estonia and Finland.
Identification of Drugs is also important for the Patient Summary service which involves a larger number of countries
In June 2021, the eHealth Network mandated the eHealth Network subgroup on semantics the mandate to review the general dataset guidelines and the ePrescription dataset guidelines; and prepare a guiding document for the development of new guidelines for datasets. The eHealth Network considers the UNICOM project as a Trusted source of information and consequently UNICOM has been providing important inputs for the update for those guidelines.
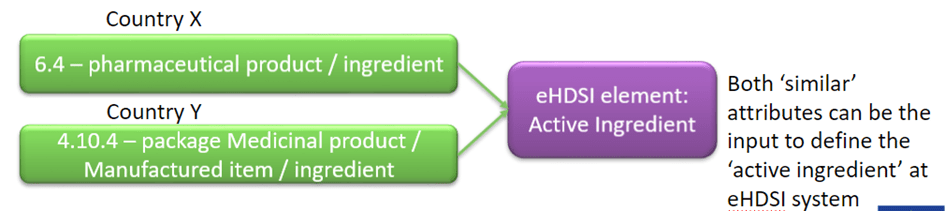
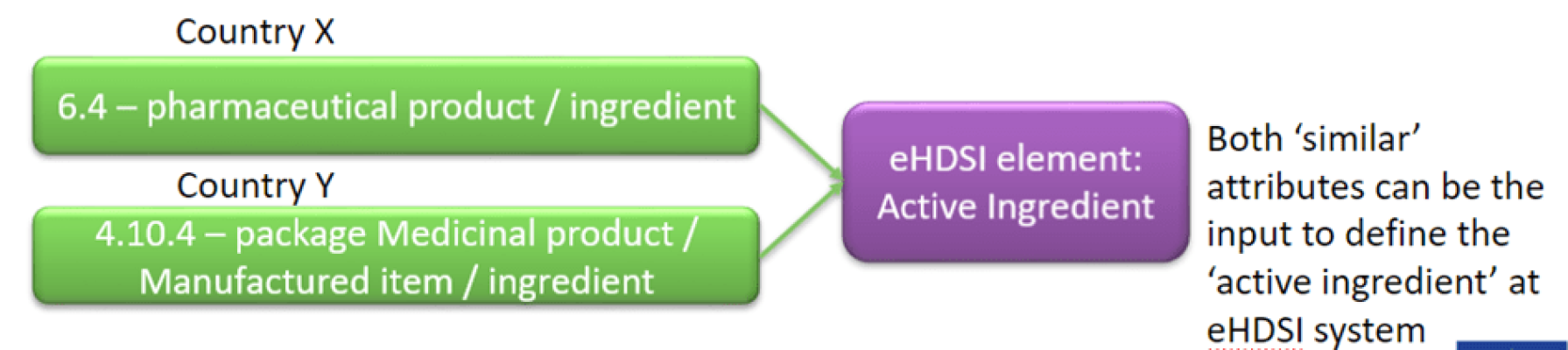
Furthemore UNICOM will be piloting IDMP implementation within the eHDSI processes and flows, by injecting «optional» IDMP Attributes in the normal flow with an objective of 10.000 ePrescriptions and 1.000 Patient Summaries which would include the IDMP attributes for all substances and products which are part of the UNICOM product Pilot list. The implementation is foreseen from the last 2023 quarter on. The definition of the minimal attribute list considering the eHDSI elements and the EMA Implementation Guideline attributes. UNICOM will indeed go beyond the EMA attributes to fulfil an eHDSI element (accordingly with each Country use)
The webinar of April 22 will present a selection of requirements identified by UNICOM WP 5, detailed in its draft deliverable D5.4, “Semantic Specifications”. Their purpose is to support semantic interoperability to be considered in local, national or regional specifications for electronic prescription, electronic dispensation and patient summaries. The requirements are a source for changes proposed to the eHealth Digital Service Infrastructure.
The presentation of this selection of requirements will be commented by other stakeholders such as from industry, national competent authorities and medicinal product dictionaries (MPD), which play a critical role in the medicinal product life cycle as described in diverse UNICOM WP1 deliveries.
Speakers:
Anderson Carmo (SPMS, WP5)
José Costa Teixeira (IHE, WP1, WP5)
Robert vander Stichele (WP1, WP9)
Frederic Bulckaen (EC)
Mathias Ghys (EC)
Panelists:
Jean-Gonzague Fontaine (GSK vaccines)
Marcello Melgara (ARIA, WP 5, WP 6 and WP7)
Moderation:
Christian Hay (GS1, ISO TC 215/WG6 and UNICOM WP1)
Robert Stegwee (CEN TC 251 and UNICOM WP1)