Join us in Montreux on September 14
Work Package 1 of the UNICOM project would like to invite you to a dedicated UNICOM day at the IHE Europe Connectathon (CAT) on Wednesday 14 September 2022 in Montreux, Switzerland.
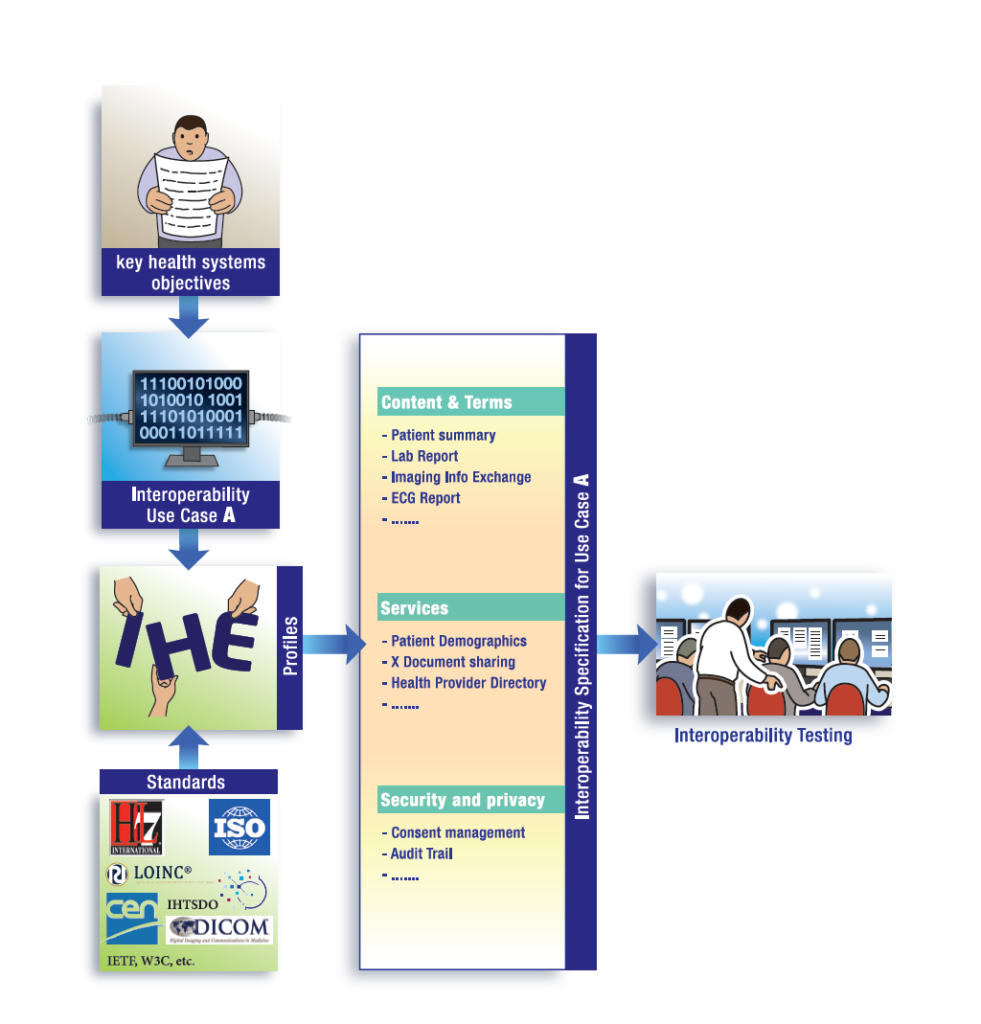
Why a dedicated UNICOM day at this year’s CAT: part of implementing IDMP is testing the interactions between different systems. An IHE Connectathon gives vendors an opportunity to test interoperability of their products in a neutral, structured and rigorous environment with peer vendors. It further gives users the benefit of conformity assessment and visibility of vendor’s products interoperability, among else. The objective is to test systems’ conformity to IHE Profiles by using validators and the interoperability between systems or simulators. We would like to familiarize the UNICOM partners and their stakeholders with the well-established and robust IHE testing, conformity and certification process, including the tooling available to support these processes. Task 1.5 of UNICOM deals with Testing and assessment and is being led by IHE Europe.
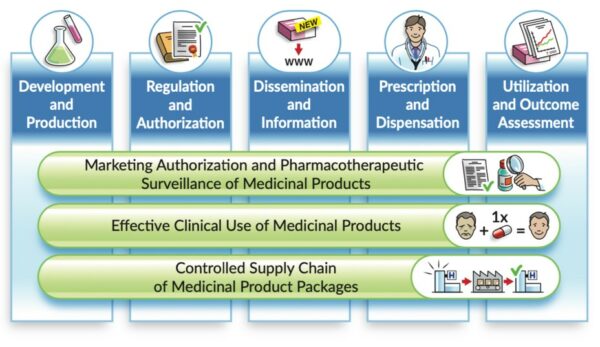
Who should attend: all stakeholders that are engaged in testing the exchange of structured data in line with IDMP-related standards and terminologies, with a special focus on the interactions with the “Regulation and Authorization” part of the IDMP landscape. In addition, we would like to specifically invite stakeholders that wish to be part of testing their implementation of structured data exchange with NCAs: industry (representing “Development and Production”) and medicinal product dictionary providers (representing “Dissemination and Information”).
IHE Pharmacy is already well-established for the latter two stages in the IDMP landscape: testing for “Prescription and Dispensation”, as well as for “Utilization and Outcome Assessment” has been in place for many years. Numerous vendors have, for instance, successfully tested their product’s implementation of the IHE profiles for Pharmacy Prescription and Pharmacy Dispense and these tests are also used for nationwide implementation in Austria (Medication List operational for several years) or Switzerland, among others.
What is a “VIP Tour”? the program provides a guided tour in the secured testing hall organised by IHE-Europe that aims to explain in a concrete way how the IHE CAT supports interoperability and conformance testing.
During this tour you will hear from IHE experts and judge for yourself about…
• The organisation of IHE and its different parts
• Gazelle: the platform that supports the tests before and during the IHE CAT
- and from the Vendors themselves. That is a unique opportunity to ask them everything you want to know and learn about the IHE testing rigor and its value for the users.
Travel and organization: For your convenience, we have decided to organize the dedicated UNICOM day on Wednesday 14 September 2022 from 09:00 to 15:00, so travellers can arrive on Tuesday and travel back home in the late afternoon and evening on Wednesday (recommended airport: Geneva; direct trains available to Montreux).
Non-UNICOM participants are required for a participation fee of 200 €, which will be invoiced by IHE-Europe before the event.
Registration link for Non-UNICOM participants: If you a UNICOM partner please use the link communicated by e-mail.
The overall programme at a glance:
| Time | Room | Speaker | |
| 9:00 | Welcome and introduction | ||
| 9:15 | Topic: Need to have neutral validation of the exchange of structured data (multistakeholders, many industries to many NCAs to many MPD providers); conformity assessment | From business need to conformance / interoperability testing: an example from IHE Pharmacy | Stephane Spahni, University Hospital Geneva, former IHE Europe co-chair |
| 10:00 | Development of profiles that allow conformance testing: workshop | Jose Costa Teixeira, former IHE Pharmacy co-chair, NN | |
| 10:45 | VIP Tour (45 min) + Coffee Break (15 min) | ||
| 11:45 | Topic: What is the role of IHE in the projects landscape | What are the challenges encountered in the EU projects | Alexander Berler, Strategy Business Development Director, IHE Catalyst, and Jürgen Brandstätter, IHE International board, member GDHP* and GCeHI* |
| 12:30 | Lunch Break | ||
| 13:30 | Topic: Real cases of Implementation | Example from various countries: IDIKA ePrescription (Greek case) + more real cases eHDSI | |
| 15:00 | End of the day |
For your information, you may as well attend the IHE Europe Experience Session on Tuesday 13 September.
*: GDHP : Global Digital Health Partnership
GCeHI : Global Consortium for eHealth Interoperability


