The eHealth Network (eHN) is a voluntary network of representatives of EU Member States. It provides a coordination platform for Member States’ competent authorities responsible for eHealth. Its major task is to cooperate and shape digital health policies and cross-border services at EU and national level, and the further development of the digital health sector. Secretarial support is provided by the European Commission, DG SANTE – Directorate-General for Health and Food Safety.
The eHN Subgroup on Semantics aims to improve interoperability by promoting the use of common standards to facilitate the exchange of patient and other health data at national and European levels, and to develop a strategy on European Semantic Interoperability. This political body is supported by the technical communities established under the EU-wide eHealth Digital Services Infrastructure (eHDSI) initiative and with which UNICOM has also established a close working relationship.
Recently, the eHN mandated its Subgroup on Semantics to revise the guidelines and standards applied for the implementation of EU-wide ePrescription services. They need to reflect new developments, particularly also the pending implementation of ISO IDMP (Identification of Medicinal Products) standards and coding systems by National Medicines Authorities and the European Medicines Agency.
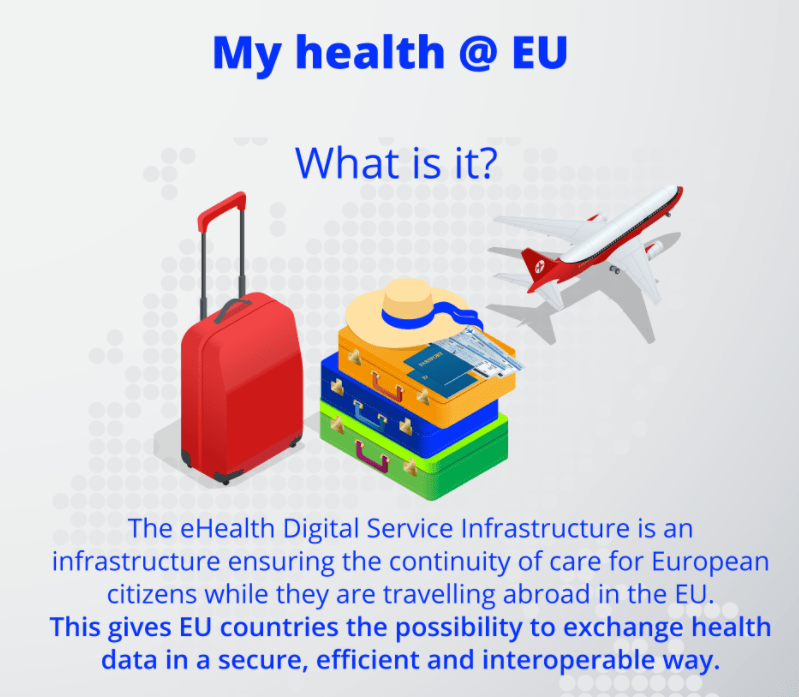
The eHDSI provides two core cross-border services for European patients: electronic exchange of Patient Summaries (PS) and of ePrescriptions (eP).
The UNICOM coordinator, Prof. Dr. Karl A. Stroetmann of empirica GmbH, has been invited to participate in the monthly meetings of the subgroup as a guest.
Based on the work of UNICOM, he is expected to contribute relevant insights for this revision process. At the recent meeting of the subgroup on 22 June, Dr. Stroetmann reported on the present status and achievements of UNICOM work relevant for the tasks of the subgroup. He focused in particular on the ongoing activities to establish a better EU-wide Substance Registration System, to implement IDMP at National Medicines Authorities, and to provide for an IDMP-conformant marketing authorisation application system for the pharmaceutical industry were explored.
As UNICOM will undertake a large pilot testing the implementation of IDMP for cross-border digital health services, already a close working relationship with the eHDSI has been established, and it was agreed to continue this exchange and UNICOM to actively contribute to the revision of the ePrescription guidelines.
Watch this video where Stefanie Weber (BfArM) detailing the role of UNICOM in the 5 years programme of the eHealth Network sub-group on semanticsDSI semantic taskforce.


