EMA Implementation Guide v2.0 is released: Let’s analyse it together on the 23rd of March during our next “Community of Expertise” meeting!

The course of IDMP implementation in Europe is strongly influenced by European Medicines Agency’s (EMA) guidance. On Monday 22 February, EMA released version 2.0 of its Implementation guide (EU IG […]
WHO Collaborating Centre for drug statistics methodology in Oslo formally agrees to collaborate with unicom

UNICOM has already welcomed the active involvement of the WHO Collaborating Centre UMC (Uppsala Monitoring Centre, Uppsala, Sweden) on questions related to the identification of substances in a pharmacovigilance context. UNICOM is now also delighted […]
Do not miss the next UNICOM Community of Expertise on Friday 19 February 2021 on the theme: UNICOM – IDMP Logical Model
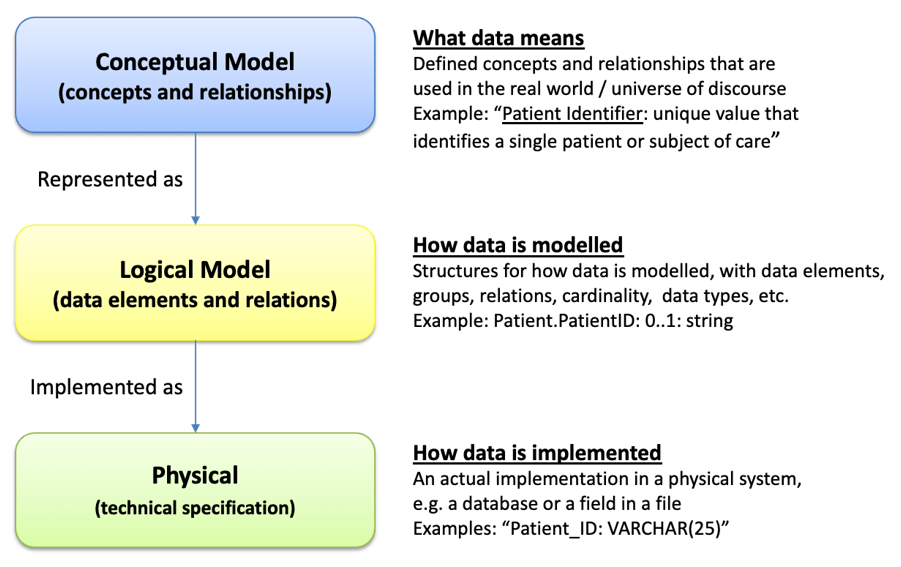
The UNICOM project has compiled and published a set of requirements for a new IDMP logical model, which shall be technology independent. The presenters will share with you the content and […]
Share your opinion on the future of EU Digital Health Apps!
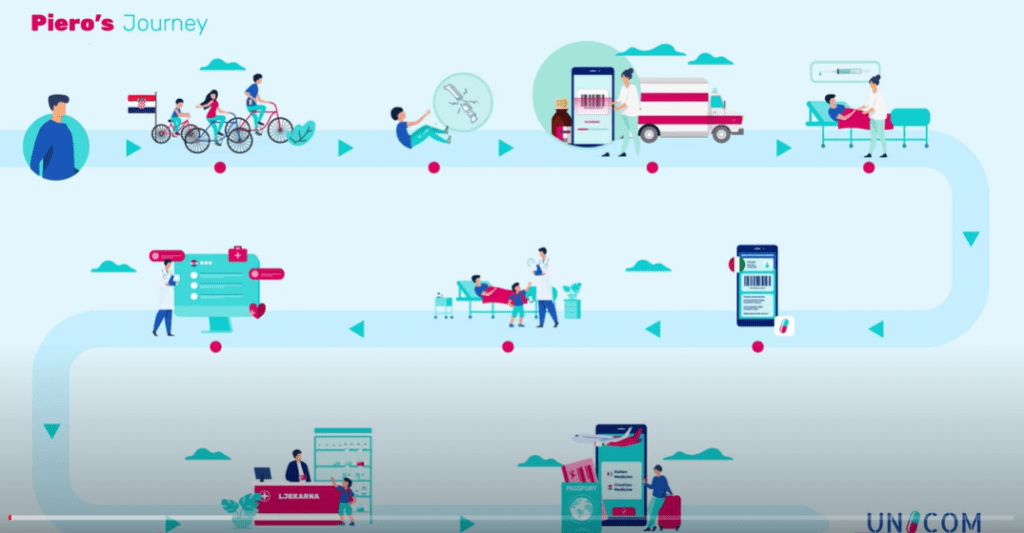
UNICOM invites you to participate in a survey of public opinions on what is important regarding patient care and empowerment when travelling outside one’s home country, specifically related to medicine. […]
The european substance reference system (EU – SRS) release strategy widely supported by the community
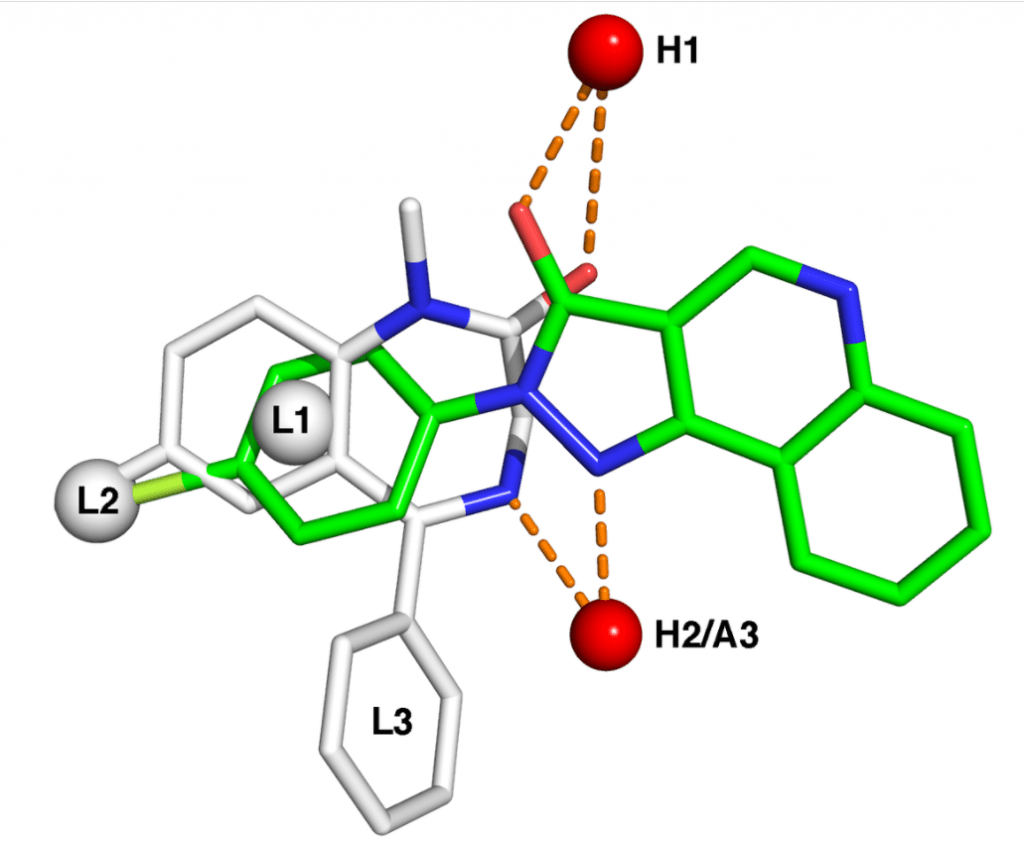
EU-SRS provides scientifically sound descriptions of substances used in medicinal products in the EU by applying regulatory standards for the identification of medicinal substances in accordance with the ISO IDMP standards. This is […]
22nd of January is the date for the next UNICOM Community of Expertise on Pharmaceutical Dose Forms
The presenters will share their perspective on how the pharmaceutical dose forms have been developed and are currently used in different situations. They will give insight in the current revision of the ISO standard, which intends to provide easier means to define world wide unique pharmaceutical dose forms. This session will also include a transatlantic perspective with the participation of the Federal Drugs Administration representative. Date: Friday […]

