Key messages from the fifth transatlantic meeting
The fifth trans-Atlantic workshop orgnanised in february 2023 was attended by representatives from UNICOM, US and EU regulators, biopharmaceutical manufacturers and technology vendors. Meeting discussions focused on global IDMP Implementation […]
EMA Product Management Service (PMS) progress webinar

Public webinar intended for business, developers and technical audiences working for industry and national competent authorities that are interested in learning more about the Product Management Service (PMS) development progress and […]
EMA WEBINAR WEEK
►You can register for the relevant Webinar by selecting the link below: SPOR and XEVMPD Data Governance – 17 April, 10:00-12:00 CET – https://ema-europa.webex.com/weblink/register/r9ca0c1044ed05282d0fe236251c99a9c Service Desk for SPOR and XEVMPD – 17 […]
Advanced FHIR training: How to import application form data into IT systems

REGISTER NOW FOR THIS EVENT Advanced FHIR training: How to track changes on a medicinal product? UNICOM is happy to invite all of you to the 4th NCA FHIR training on […]
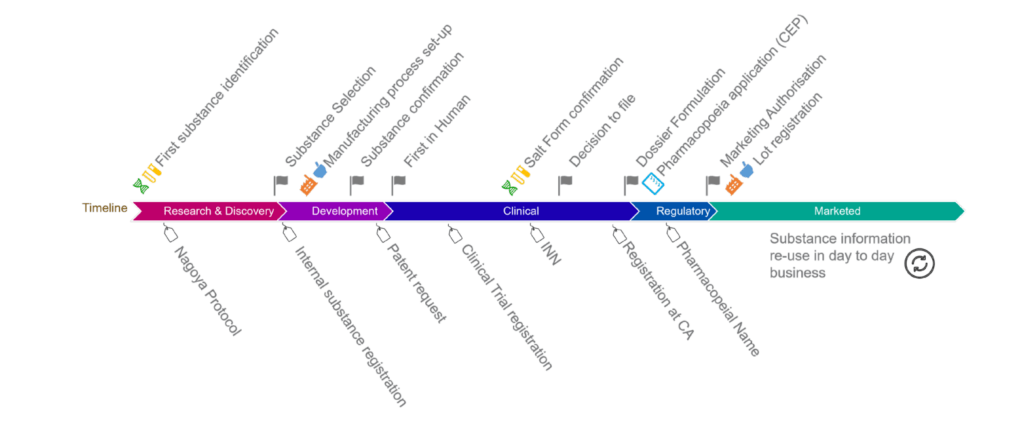
EU-SRS is live at EMA: a major IDMP related milestone reached!

It is celebration day today (Jan 25) in Amsterdam at EMA headquarters. UNICOM is very pleased to announce that EU-SRS (the European Substances Registration System) is operational at EMA. The […]
Intensive preparation and training to prepare the release by EMA of variations form for human medicinal products in october 2022
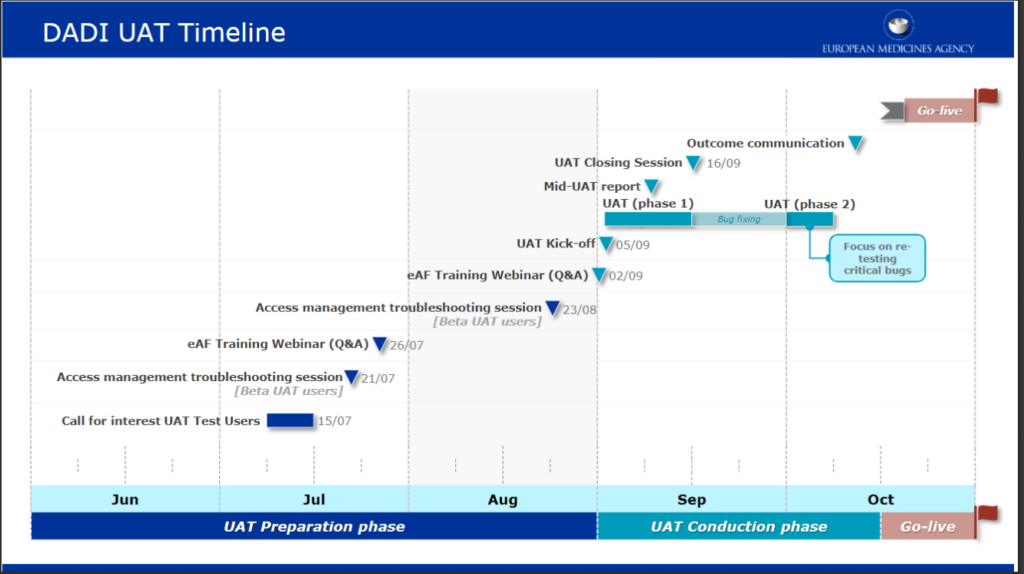
The Digital Application Dataset Integration (DADI) Network project will replace PDF electronic application forms (eAF) used for regulatory submissions with web-forms, making the future form-filling and submission-handling process more efficient. […]

