Norway, Belgium, Greece, Italy, Finland and the USA collaborate with UNICOM to provide a limited (4 substances) but complete IDMP/FHIR data set.
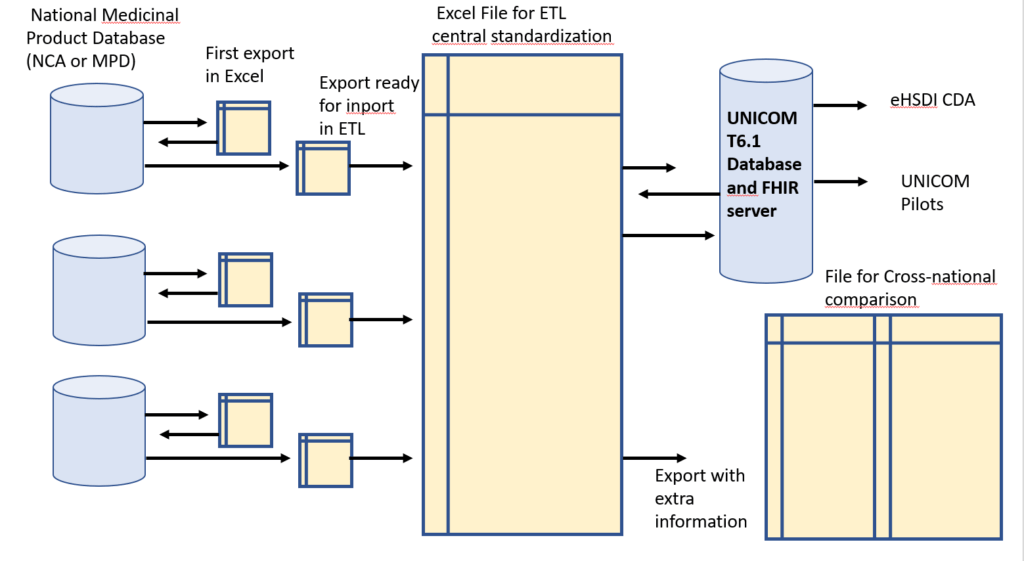
The process consists in collecting Data “AS IS”, and doing a central standardization to EDQM, limited to the minimal attribute list (variables relevant for pilots) and limited to 4 single […]
Discover what can IDMP do to improve the quality of drugs dictionaries and support best ETL practices during our Community of Expertise on February 25 at 15.00 CET
Using IDMP in Medicinal Product Dictionary This webinar will present IDMP as driver for Medicinal Product Data (MPD) to increase quality of MPD data, make use of one trusted source, […]

