EMA Product Management Service (PMS) progress webinar

Public webinar intended for business, developers and technical audiences working for industry and national competent authorities that are interested in learning more about the Product Management Service (PMS) development progress and […]
Norway, Belgium, Greece, Italy, Finland and the USA collaborate with UNICOM to provide a limited (4 substances) but complete IDMP/FHIR data set.
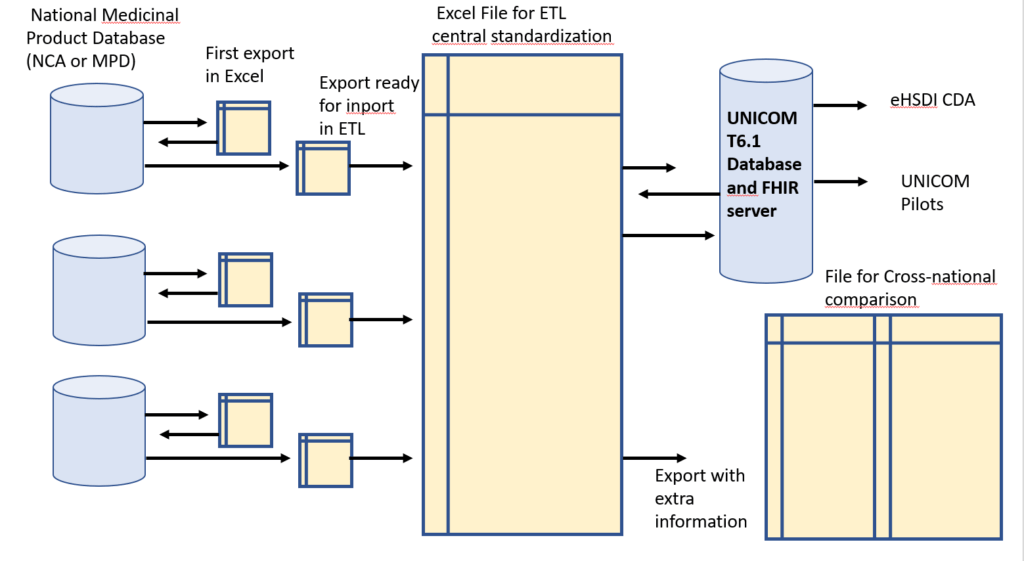
The process consists in collecting Data “AS IS”, and doing a central standardization to EDQM, limited to the minimal attribute list (variables relevant for pilots) and limited to 4 single […]
GERMAN SOCIETY FOR REGULATORY AFFAIRS (DGRA) Annual Congress
Georg NEUWIRTHER will present UNICOM on May 5th at 10.00 AM. GERMAN SOCIETY FOR REGULATORY AFFAIRS (DGRA) The German Society for Regulatory Affairs e.V. is a scientific specialist society in […]
UNICOM day IHE Connectathon in Rennes

In the context of the IHE-Europe Connectathon WEEK 2023 | Connectathon in Rennes France and in parrallel to the French eHealth week. Looking across the landscape of IDMP implementation, the […]
Putting resources developed by UNICOM to the test: Preparing the UNICOM IHE Connecthathon
This webinar is targeted at all European NCAs, eHealth competence centers and Industry (EMR, MPDs, HCP and Patient facing Apps). It will present and discuss the approach porposed by IHE […]
Knowledge transfer Webinar: Norway (NOMA) in focus

Administrable products : Based on relationship between manufactured and administrable dose form, – NoMA’s solution to meet needs in clinical practice. REGISTER
