Norway, Belgium, Greece, Italy, Finland and the USA collaborate with UNICOM to provide a limited (4 substances) but complete IDMP/FHIR data set.
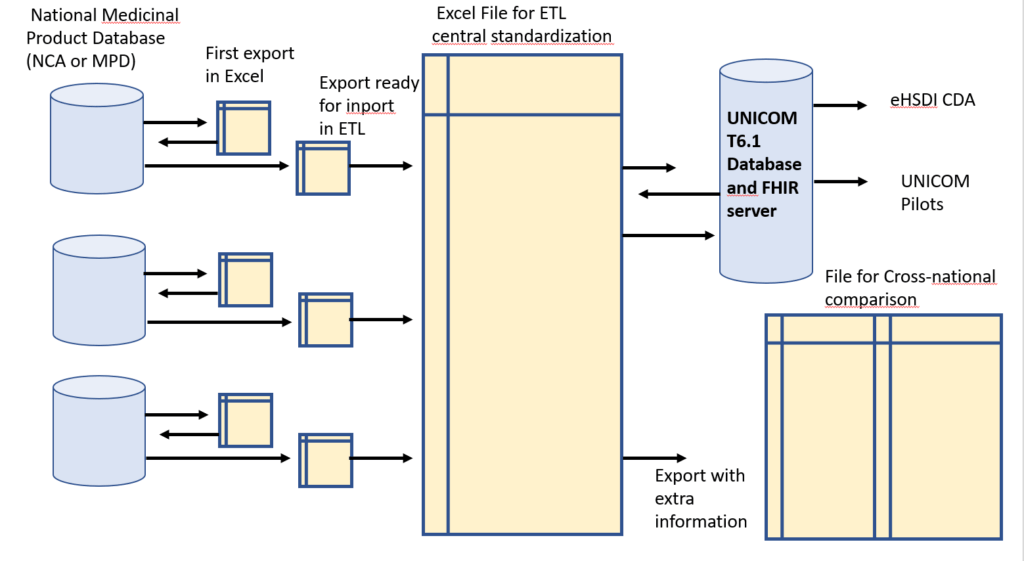
The process consists in collecting Data “AS IS”, and doing a central standardization to EDQM, limited to the minimal attribute list (variables relevant for pilots) and limited to 4 single […]
EU-SRS is live at EMA: a major IDMP related milestone reached!

It is celebration day today (Jan 25) in Amsterdam at EMA headquarters. UNICOM is very pleased to announce that EU-SRS (the European Substances Registration System) is operational at EMA. The […]
UNICOM Community of Expertise: SUBSTANCE and EU-SRS
The 4th of November a Community of Expertise will be held for which you have been invited. The presenters will be Annet Rozema, Mirjam Keulen & Marcel Hoefnagel from CBG, […]
UNICOM Community of Expertise: Vaccine challenges – cleansing, confidentiality, and vaccine naming
The need for global identification of vaccines has been recognized in the Global Vaccines Initiative, addressing the global surveillance of vaccine effectiveness and risks. This has particular consequences for the […]
Global Vaccines Initiative concludes that globalization of substance management is feasible

While the booster vaccination against Covid-19 is proceeding at full speed in most European countries and worldwide, the question of the correct identification and global alignment on vaccines substances is still […]
