Knowledge Transfer Webinar: Germany (BFarm) in focus
UNICOM Community of Expertise: Putting UNICOM ressources to the test

Across the UNICOM project we have been actively developing a number of standards-based resources, including FHIR Implementation Guides, a Product Lifecycle Management portal (with EMA), an IDMP database, a substitution […]
IRISS TG meeting
Unicom developments around IDMP from a NCA (AGES) perspective by George Neuwirther IDMP Ontology by Heiner Oberkampf and Raphael Sergent EU-SRS by Annet Rozema
Key messages from the fifth transatlantic meeting
The fifth trans-Atlantic workshop orgnanised in february 2023 was attended by representatives from UNICOM, US and EU regulators, biopharmaceutical manufacturers and technology vendors. Meeting discussions focused on global IDMP Implementation […]
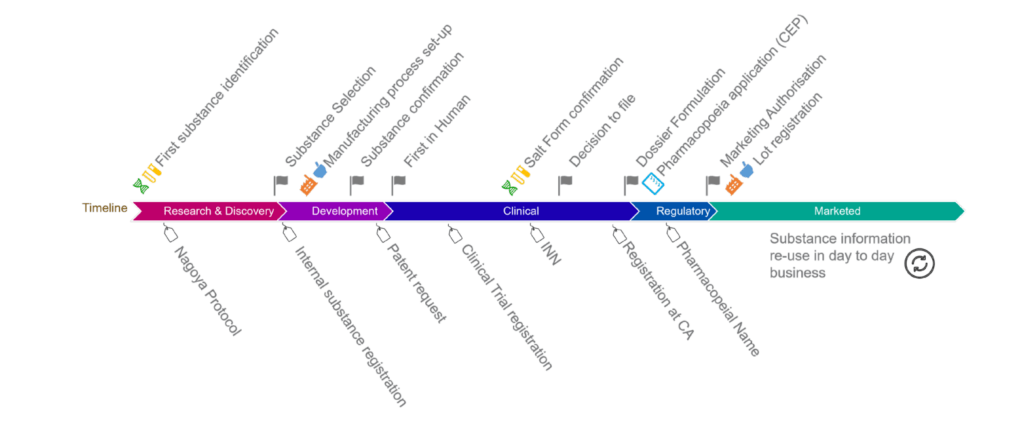
UNICOM Community of Expertise: Global alignment on substances based on the ISO IDMP and the SRS software

A report on progress and challenges from the Global IDMP Working Group (GIDWG), about substances. The presentation will give an overview of the GIDWG work on global alignment of substances, […]
A vast majority of European National Competent Authorities engaged in IDMP implementation

The Swedish Medicinal Products Agency is leading the UNICOM work package dedicated to IDMP implementation within National Authorities competent for market authorisation. Sweden is currently ensuring the Presidency of the […]
