Watch or re-watch UNICOM session: Medicines data integration for clinical use: New implementation patterns”

Robert Van Der Stichele (I~HD) moderated the session “Medicines data integration for clinical use: New implementation patterns“. Today, e-prescribing systems are spreading steadily across Europe. While they are important, these […]
3rd GIDWG Stakeholder Group Meeting took place in Delft (Holland)
UNICOM is engaged with the Global IDMP Working Group (GIDWG), created by EMA, FDA and WHO-UMC. The 3rd GIDWG Stakeholder Group Meeting took place this week and the results are […]
Key messages from the fifth transatlantic meeting
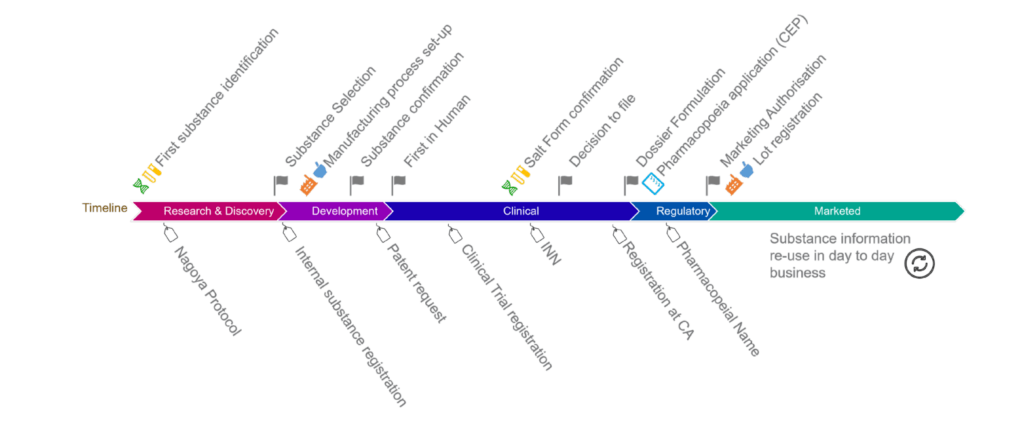
The fifth trans-Atlantic workshop orgnanised in february 2023 was attended by representatives from UNICOM, US and EU regulators, biopharmaceutical manufacturers and technology vendors. Meeting discussions focused on global IDMP Implementation […]
A vast majority of European National Competent Authorities engaged in IDMP implementation

The Swedish Medicinal Products Agency is leading the UNICOM work package dedicated to IDMP implementation within National Authorities competent for market authorisation. Sweden is currently ensuring the Presidency of the […]
Norway, Belgium, Greece, Italy, Finland and the USA collaborate with UNICOM to provide a limited (4 substances) but complete IDMP/FHIR data set.
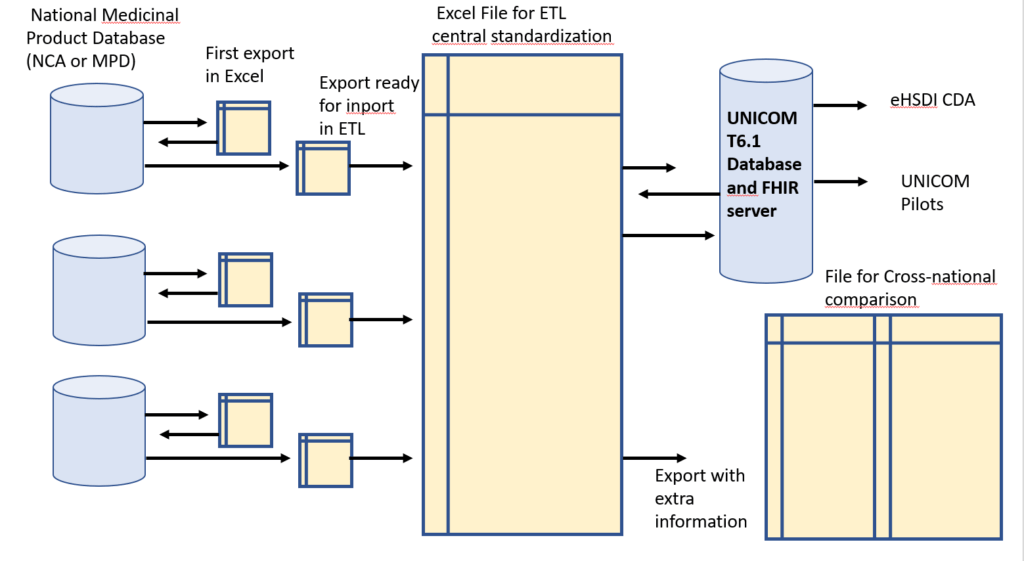
The process consists in collecting Data “AS IS”, and doing a central standardization to EDQM, limited to the minimal attribute list (variables relevant for pilots) and limited to 4 single […]
EU-SRS is live at EMA: a major IDMP related milestone reached!

It is celebration day today (Jan 25) in Amsterdam at EMA headquarters. UNICOM is very pleased to announce that EU-SRS (the European Substances Registration System) is operational at EMA. The […]

