Intensive preparation and training to prepare the release by EMA of variations form for human medicinal products in october 2022
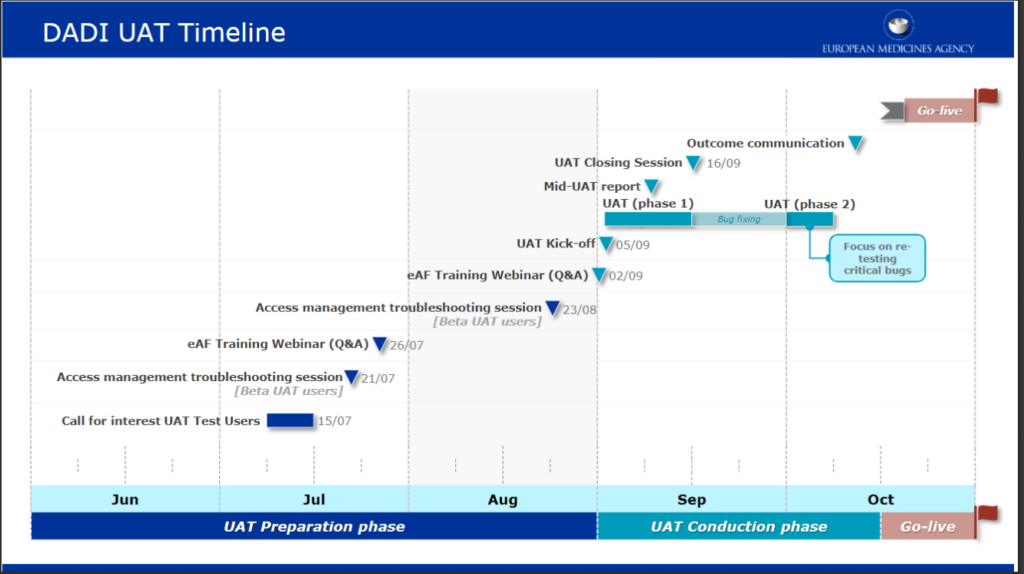
The Digital Application Dataset Integration (DADI) Network project will replace PDF electronic application forms (eAF) used for regulatory submissions with web-forms, making the future form-filling and submission-handling process more efficient. […]
A new scientific article published this week in the Journal Applied Science advocates for the use of the EDQM Standard Terms Database for Pharmaceutical Dose Forms

In the context of the UNICOM project, researchers from the European Institute for Innovation through Health Data analysed the Standard Terms for Pharmaceutical Dose Forms from the European Directorate for Quality […]

