ALL PUBLIC KNOWLEDGE TRANSFER WEBINARS

COUNTRY/Topic DATE VIDEO SLIDES IRELAND : Lesson learnt June 21 2022 Link Link SWEDEN : strategy, projectmanagement and developmentofIT-systems October 13 2022 Link Link ESTONIA : overview (demo) of the new IDMP compliant […]
A vast majority of European National Competent Authorities engaged in IDMP implementation

The Swedish Medicinal Products Agency is leading the UNICOM work package dedicated to IDMP implementation within National Authorities competent for market authorisation. Sweden is currently ensuring the Presidency of the […]
NOMA and WHO-UMC are testing the PhPID generation
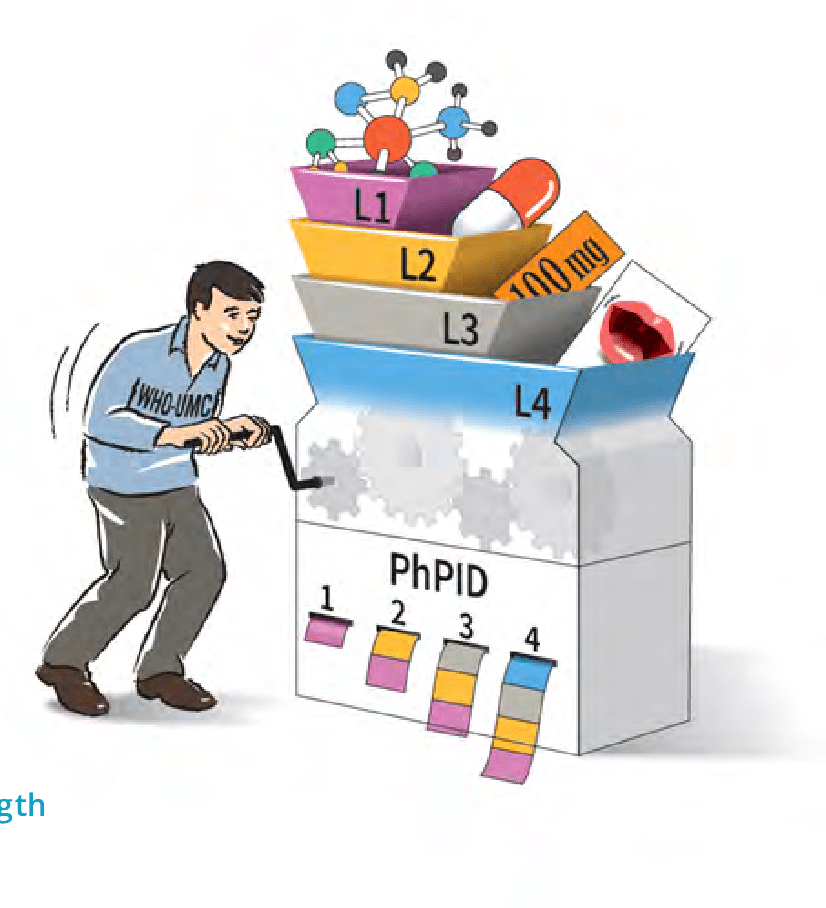
The Norwegian Medicines Agency is very much aware of its role of interoperabilty enabler and has initiated in 2019 the SAFEST project which aims at the distribution of interoperable medicinal […]
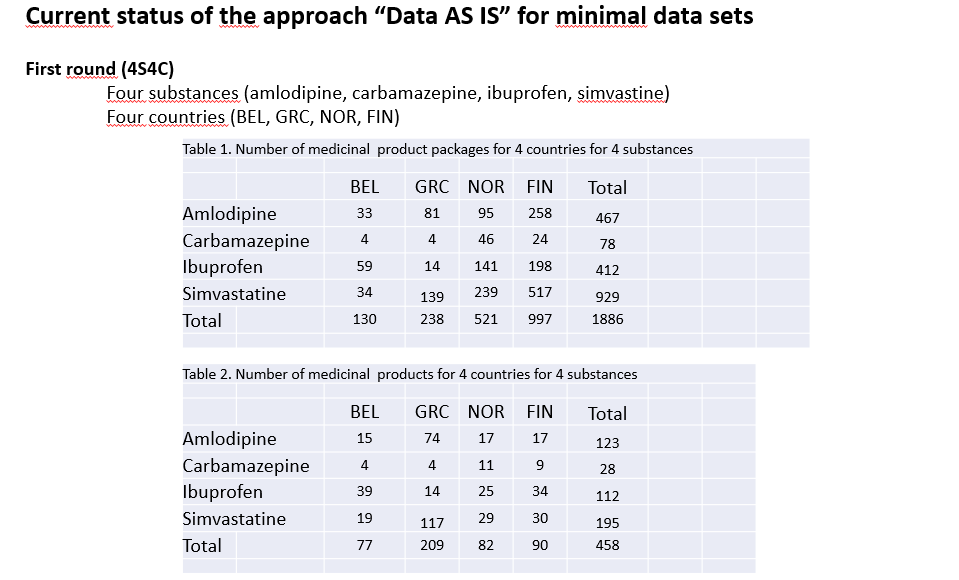
Norway, Belgium, Greece, Italy, Finland and the USA collaborate with UNICOM to provide a limited (4 substances) but complete IDMP/FHIR data set.
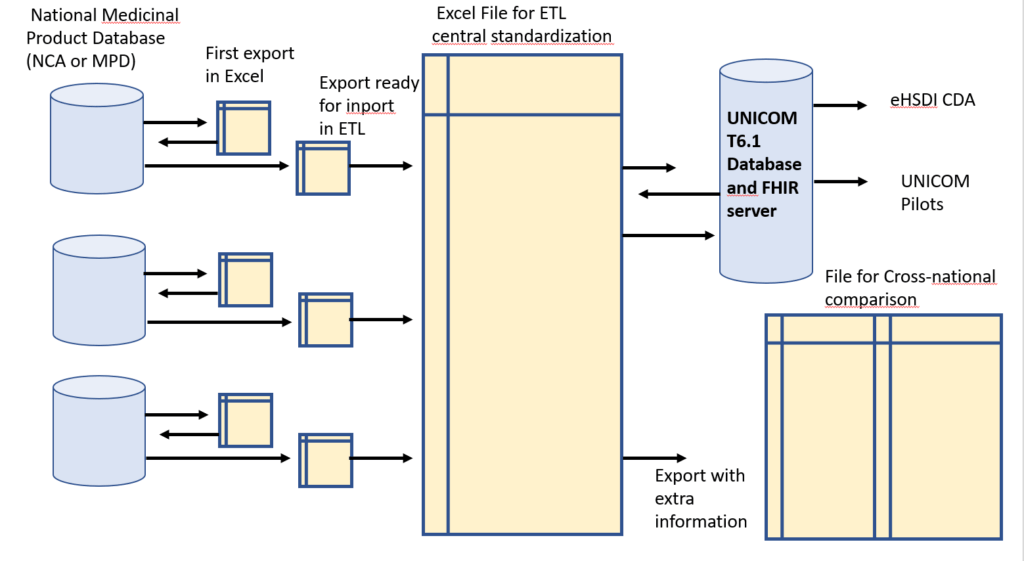
The process consists in collecting Data “AS IS”, and doing a central standardization to EDQM, limited to the minimal attribute list (variables relevant for pilots) and limited to 4 single […]
EU-SRS is live at EMA: a major IDMP related milestone reached!

It is celebration day today (Jan 25) in Amsterdam at EMA headquarters. UNICOM is very pleased to announce that EU-SRS (the European Substances Registration System) is operational at EMA. The […]
UNICOM creates a momentum for IDMP implementation in Greece during the Panhellenic Conference 2022
The Panhellenic Conference 2022 on finances and health policies is now over and UNICOM is very happy about the discussions, the results achieved and the next steps proposed. The participants […]

