First electronic product information (ePI) published

The Heads of Medicines Agencies (HMA), the European Commission (EC), the European Medicines Agency (EMA) and a number of pharma companies have published for the first time electronic product information (ePI) for selected human medicines harmonised across the […]
3rd GIDWG Stakeholder Group Meeting took place in Delft (Holland)
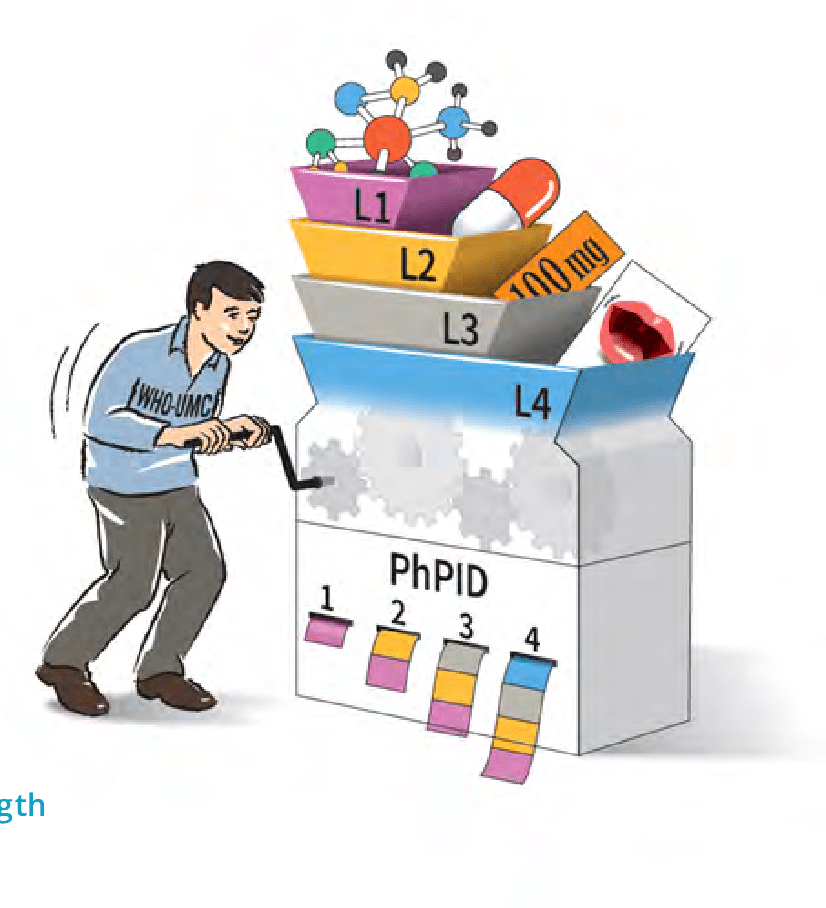
UNICOM is engaged with the Global IDMP Working Group (GIDWG), created by EMA, FDA and WHO-UMC. The 3rd GIDWG Stakeholder Group Meeting took place this week and the results are […]
A PROMISING MPs substitution component!
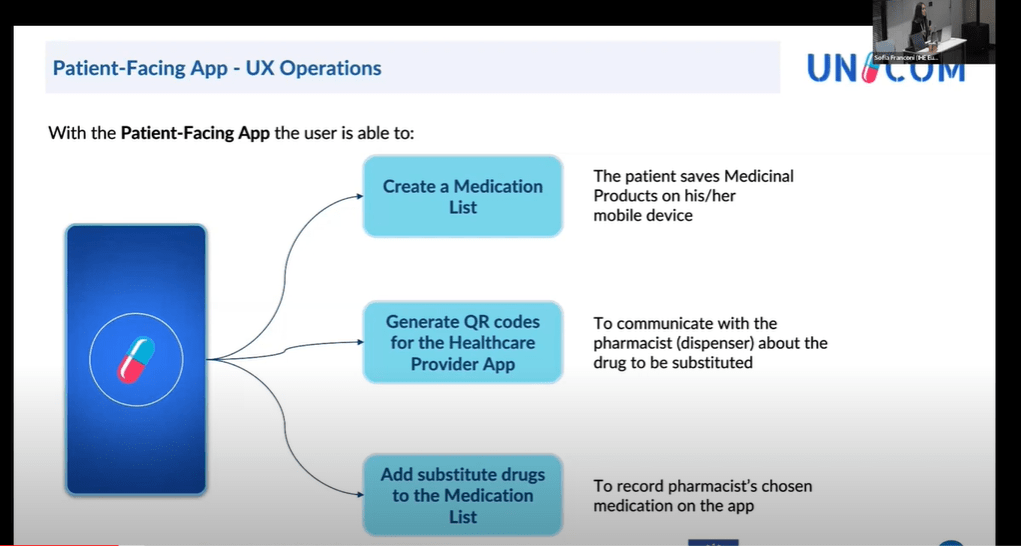
Currently substitution in MyHealth@EU is limited to a “selection” of fully equivalent medicinal products. The potentialities of “substitution methodology”, proposed by UNICOM should be considered by the MyHealth@EU ePrescription Cluster […]
Join us in Ghent (Belgium) on November 29 – December 1

UNICOM has joint forces with two partner organisations (I~HD and EHTEL) to organise a very informative week in Ghent (Belgium) from November 29th till December 1st. We have two main objectives […]
Linking IDMP identifiers and ATC: What could be the benefits ?

On October 3, Dr Robert VanderStichele was representing UNICOM during a meeting organised by the WHO Collaborating Centre for Drug Statistics Methodology. He provided important insights on the opportunities for […]
Key messages from the fifth transatlantic meeting
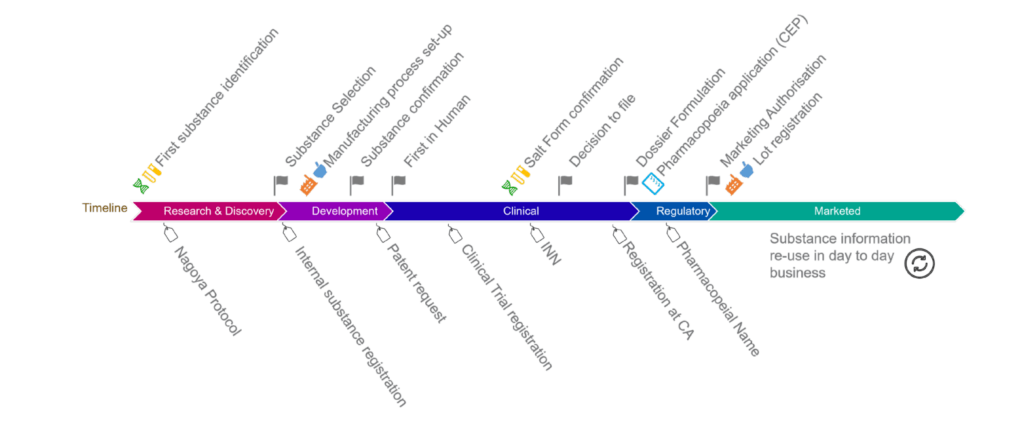
The fifth trans-Atlantic workshop orgnanised in february 2023 was attended by representatives from UNICOM, US and EU regulators, biopharmaceutical manufacturers and technology vendors. Meeting discussions focused on global IDMP Implementation […]

