MIE 2022 in Nice: “Challenges of trustable AI and added-value on health”
An UNICOM abstact has been accepted. UNICOM is thus proud that it will be part of the official programme with a full oral presentation. Lin to the event: https://mie2022.org/
An UNICOM abstact has been accepted. UNICOM is thus proud that it will be part of the official programme with a full oral presentation. Lin to the event: https://mie2022.org/
Those webinars are meant to facilitate an early and detailed cross-fertlisation between European National Competent Authorities. Ireland (HPRA) will be presnting its current sttaus on its road toward IDMP implementation. All staff of all European agencies are warmly welcome to attend. If you have not received an invitation but would like to attend, please send […]
The Croatian Medicinal products and medical devices Agency (HALMED) will be presenting its roadmap to IDMP implemenation at this event. See: https://www.iaria.org/conferences2022/eTELEMED22.html
The Digital Application Dataset Integration (DADI) Network project team is pleased to announce the publication of the first version of the eAF Portal guide to registration. The guide aims to support the users of the eAF Portal in completing the registration steps before accessing the platform. Most of these steps are independent from the eAF Portal and […]
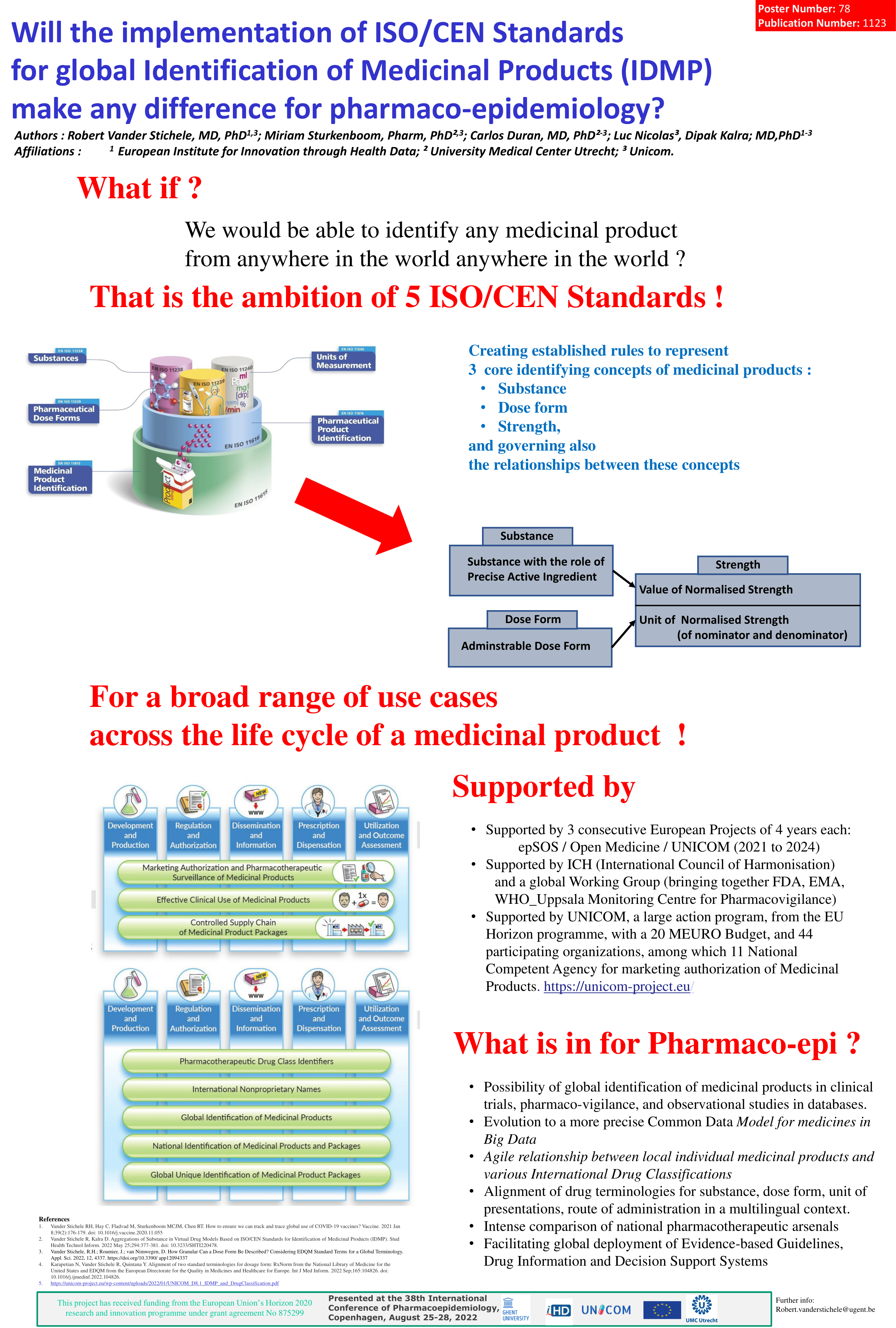
Advancing Pharmacoepidemiology and Real-World Evidence for the Global Community UNICOM WP8 leader Robert Vanderstichele will be presnting a poster on the the theme: Will the implementation of ISO/CEN Standards for global Identification of Medicinal Products (IDMP) make any difference for pharmaco-epidemiology ? Vander Stichele RH, Sturkenboom MCJM, Duran C, Nicolas L, Kalra D, Fladvad M.
The next UNICOM Community of Expertise is scheduled on Friday 26 August 2022: In collaboration with the Gravitate Health project and the Vulcan Accelerator ePI project, UNICOM has contributed to a track at the May HL7 FHIR Connectathon. The scenario being developed was on a travelling lady needing treatment to a recurring condition, for which […]
The Digital Application Dataset Integration (DADI) Network project team is pleased to announce the publication of the first version of the eAF Portal guide to registration. The guide aims to support the users of the eAF Portal in completing the registration steps before accessing the platform. Most of these steps are independent from the eAF Portal and […]
The purpose is to familiarize the UNICOM partners and impacted stakeholders with the well-established and robust IHE testing and certification process, including the tooling available to support these processes. The session will have a special focus on the interactions between Regulation and Authorization authorities - industry (representing Development and Production) and medicinal product dictionary providers […]
The International Pharmaceutical Federation (FIP) is the global body representing pharmacy and pharmaceutical sciences. Through our 147 national organisations, academic institutional members and individual members, we represent over four million pharmacists and pharmaceutical scientists around the world. Congress Theme: Pharmacy united in the recovery of health care At this critical time, when the challenges presented […]
The 4th of November a Community of Expertise will be held for which you have been invited. The presenters will be Annet Rozema, Mirjam Keulen & Marcel Hoefnagel from CBG, The Netherlands, UNICOM WP 2. Substances have been the topic of the UNICOM Community of Expertise before and this is not a surprise as substances […]
Organized by ISfTeH A one-of-a-kind meeting to discuss successful business models, opportunities in emerging markets, the challenges, and solutions for cybersecurity and international regulatory navigation.* The International Society for Telemedicine and eHealth (ISfTeH) is a federation of 45 national professional associations in the Telemedicine and eHealth space globally. The society also has institutional, corporate, and […]
The 2022 edition of pHealth will emphasize the interrelated aspects of pHealth, i.e. advanced digital health ecosystems. In that context, mobile technologies, micro-nano-bio smart systems, bio-data management and analytics, machine learning, artificial intelligence and robotics for personalized health, the Health Internet of Things (HIoT), systems medicine, public health and virtual care. UNICOM WP1 intends to […]
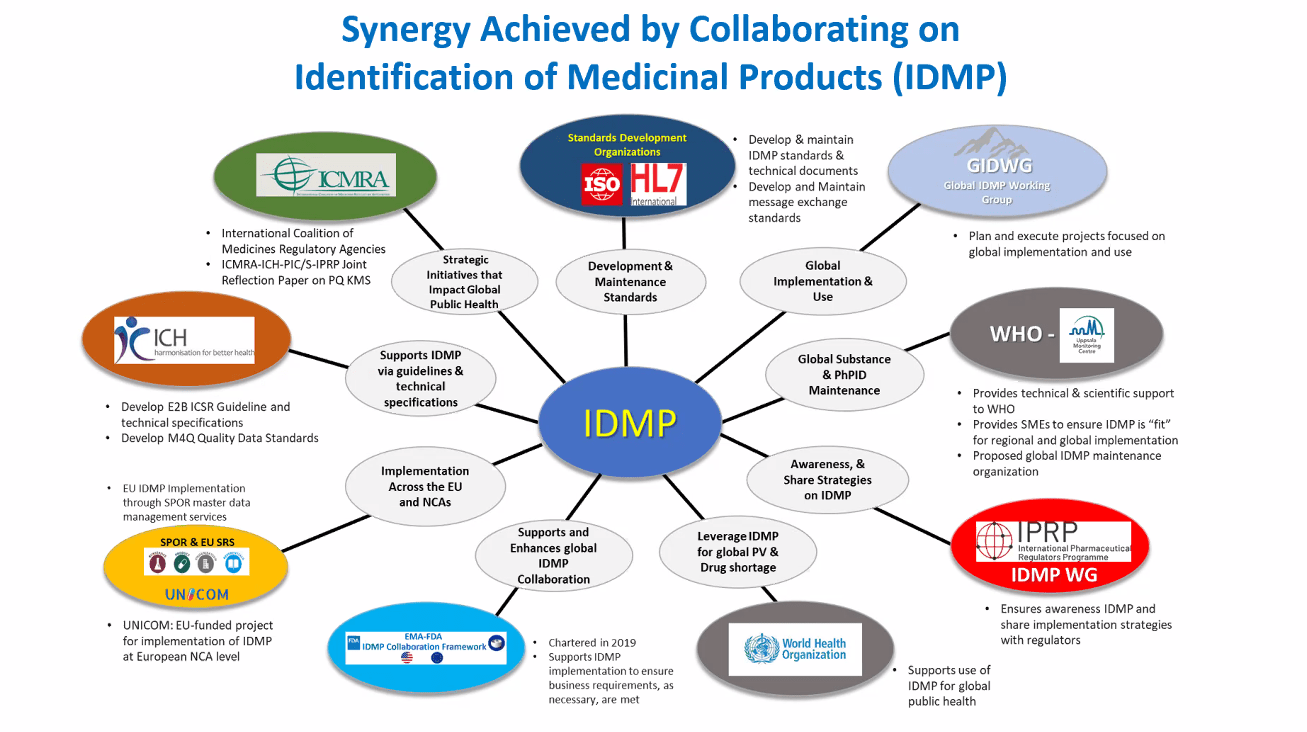
The UNICOM trans-Atlantic meetings are coordinated by CTADHL, a UNICOM partner formally tasked for trans-Atlantic outreach by UNICOM to include promotion and adoption of ISO IDMP across the medicinal product lifecycle. Although UNICOM is an EU initiative, it strongly considers collaboration and exchange with North American stakeholders as part of its remit to actively promote […]
Take part today at 13.00 in the 5TH ePI Vulcan accelerator discussion with a joint input of both UNICOM - Up-scaling the global univocal identification of medicines and Gravitate Health -> All the detailed information about the track can be found here: https://lnkd.in/eD8x3TQu This is the 5th event in the series of companion events to Vulcan ePI Project […]
Description: The landscape of use of medication data is quite broad and diverse. When looking at standards for the exchange of medication data, including the terminology mappings needed to cross the borders between the different domains, it is important to identify the original sources of the data being exchanged. These sources are the key first […]
This event is a webinar for industry and national competent authorities’ stakeholders wishing to learn more about data migration activities from SIAMED and xEVMPD databases to PMS
UNICOM is happy to announce a new series of IDMP implementation related training and knowledge transfer webinars in 2023. Considering the recent progress with EMA eAF we believe that it is important that all European NCAs acquire without delay a better understanding of the FHIR standard and its relevance in relationship to a wide IDMP […]
HL7 FHIR® is the communication standard for exchanging healthcare data being adopted worldwide. Past announcements from the FDA and current developments by the European Union (EHDS, EMA) and important and strategic projects such as UNICOM and Gravitate Health all prioritize the IDMP suite of standards as global reference and FHIR as underlying data transmission standard. […]
REGISTER NOW FOR THIS EVENT

Agenda : 25’ Results of the FAMHP IDMP gap analysis: 1. EMA : deviations (more, less, different) versus ISO IDMP documents (of 2012 and 2017, whichever is more recent) 2. FAMHP MPM/DTS : differences with the EMA data model 3. FAMHP approach to narrow the gaps 10’ QUESTIONS 15’ SAM / ePrescription : dataflow from BE […]
►You can register for the relevant Webinar by selecting the link below: SPOR and XEVMPD Data Governance - 17 April, 10:00-12:00 CET - https://ema-europa.webex.com/weblink/register/r9ca0c1044ed05282d0fe236251c99a9c Service Desk for SPOR and XEVMPD - 17 April, 14:00-16:00 CET - https://ema-europa.webex.com/weblink/register/r1552802c0646096c101b5e62b25dfa5d Referentials Management Service (RMS) - 18 April, 10:00-12:00 CET - https://ema-europa.webex.com/weblink/register/r46516995ff51d5f4383d95f05cac8e38 Organisation Management Service (OMS) - 18 April, 14:00-16:00 - https://ema-europa.webex.com/weblink/register/r834ea26426a57529292e0dcffc96cb5a Substance Management […]
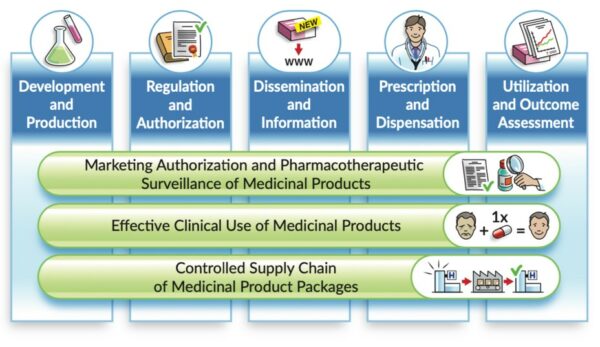
Description: Referring to the landscape of medicinal product data – from “research and development” to “utilisation and outcome assessments”- the vision is that the essential IDMP identifiers remain used and unchanged along that life cycle. This Community of Expertise (CoE) will provide opportunity : for industry to present their perspective and needs, with large international […]