


How digitalisation can support the regulatory business process Britt Vermeij, Vice-Chair of the Regulatory and Scientific Affairs Committee Medicines for Europe, TEVA - A coherent data strategy as foundation to […]

UNICOM – Pharmaceutical Dose Forms The presenters will share their perspective on how the pharmaceutical dose forms have been developed and are currently used in different situations. They will give […]

The objectives of this Workshop are to present in some detail the history, and particularly the present organisational structure, data flows and technical details of the ePrecription/eDispensation and Patient Summary […]

The objectives of this Workshop are to present in some detail the history, and particularly the present organisational structure, data flows and technical details of the ePrecription/eDispensation and Patient Summary […]
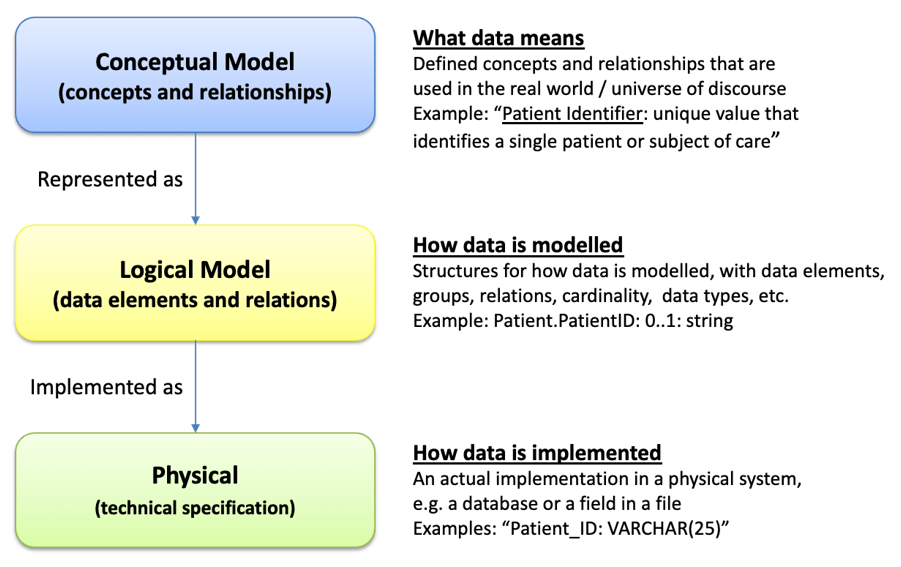
The UNICOM project has compiled and published a set of requirements for a new IDMP logical model, which shall be technology independent. Currently, the IDMP data model is described by partial logical models across the different standards making up the IDMP set of standards and by HL7 v3 based specifications in some of the technical specifications. The ISO group of experts will take these requirements as input for the development of a new ISO IDMP logical model. Our discussion of the requirements will also be made available to the ISO experts, as they join the conversation.
Joint Initiative Council Open Forum - Morning Session Transcending national boundaries and organizations, the Joint Initiative Council’s standards development organizations work collaboratively to provide global, coordinated—not competitive—standards that address real-world […]

Transcending national boundaries and organizations, the Joint Initiative Council’s standards development organizations work collaboratively to provide global, coordinated—not competitive—standards that address real-world healthcare issues. In its recently released white paper, […]
The current status of UNICOM work and expected results is presented by C.Hay (WP1) at the meeting of the International pharmaceutical regulators programme (http://www.iprp.global/home)
Preliminary Program Welcome – Agenda State of play with Vaccination Standards in Europe: an overview Christof Gessner, HL7 Europe Board of Directors, HL7 Germany What is the state of play […]
UNICOM – EMA Implementation Guide v2.0 The course of IDMP implementation in Europe is strongly influenced by European Medicines Agency's (EMA) guidance. On Monday 22 February, EMA released version 2.0 of […]
Understand the issues at stake and make your voice heard by attending the first UNICOM event targeted at national and European healthcare professionals and patients' organisations Download PDF Register NOW
The importance of medicinal product dictionaries (MPDs) is highlighted by two concrete examples. MPDs bring together the information on medicinal products from NCA and a variety of sources. Making sure […]