
First electronic product information (ePI) published
The Heads of Medicines Agencies (HMA), the European Commission (EC), the European Medicines Agency (EMA) and a number of pharma companies have published for the first time electronic product information (ePI) for selected human medicines harmonised across the
3rd GIDWG Stakeholder Group Meeting took place in Delft (Holland)
UNICOM is engaged with the Global IDMP Working Group (GIDWG), created by EMA, FDA and WHO-UMC. The 3rd GIDWG Stakeholder Group Meeting took place this week and the results are
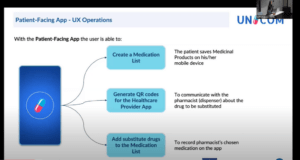
A PROMISING MPs substitution component!
Currently substitution in MyHealth@EU is limited to a “selection” of fully equivalent medicinal products. The potentialities of “substitution methodology”, proposed by UNICOM should be considered by the MyHealth@EU ePrescription Cluster

Join us in Ghent (Belgium) on November 29 – December 1
UNICOM has joint forces with two partner organisations (I~HD and EHTEL) to organise a very informative week in Ghent (Belgium) from November 29th till December 1st. We have two main objectives

Linking IDMP identifiers and ATC: What could be the benefits ?
On October 3, Dr Robert VanderStichele was representing UNICOM during a meeting organised by the WHO Collaborating Centre for Drug Statistics Methodology. He provided important insights on the opportunities for
Key messages from the fifth transatlantic meeting
The fifth trans-Atlantic workshop orgnanised in february 2023 was attended by representatives from UNICOM, US and EU regulators, biopharmaceutical manufacturers and technology vendors. Meeting discussions focused on global IDMP Implementation

