
UNICOM present in Panhellenic Congress to support IDMP implementation in Greece
The Panhellenic Congress on Economics and Health Policy 2022 is taking place in Athens on December 12-15, 2022. The focus of the 2022 Congress is on “Sustainability and resilience: Securing

Kicking off the work on Marketing Authorization Applications (eAF MAA)
The EMA eAF electronic application for Variation Forms went live for Centralised Authorised Products on November 4. This is a first major step forward in the effort to digitalise business
New EMA web-based Human Variations electronic Application Form has gone live on November 4 bringing far-reaching effects
The web-based Human Variations electronic application form (eAF) for centrally authorised products (CAPs) is available for use since November 4 on the new Product Lifecycle Management (PLM) Portal hosted by
Time for a new Community of Expertise on November 4 focusing on EU-SRS: the system created to manage substances in Europe
When ? The 4th of November from 15.00 till 16.30 CEST Substances have already been the topic of a previous UNICOM Community of Expertise in August 2021 and this is

NoMA, the Norwegian Medicines Agency, to provide a substantial amount of data for the UNICOM FHIR® IDMP server
Norway is not an EU member State but is often a key player when innovation is at stake. NoMA is indeed one of the most proactive UNICOM partners and is
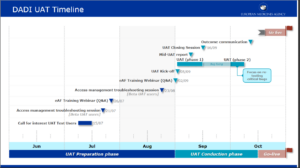
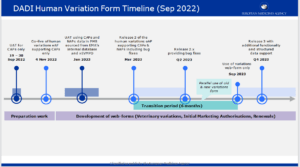
Intensive preparation and training to prepare the release by EMA of variations form for human medicinal products in october 2022
The Digital Application Dataset Integration (DADI) Network project will replace PDF electronic application forms (eAF) used for regulatory submissions with web-forms, making the future form-filling and submission-handling process more efficient.

