Sharing UNICOM results with the US audience
The webinar will be organised by UNICOM partner HARVARD MEDICAL SCHOOL and has as key objective to update the American interested audience about UNICOM results and discuss in particular some […]
A clinical value chain workshop in Brussels confirms the strategic added-value of IDMP
UNICOM hosted in March 2024 a workshop that aimed to engage stakeholders in discussions about the advantages,challenges, obstacles, and timelines related to the implementation and utilisation of IDMP in the […]
Watch or re-watch UNICOM session: Medicines data integration for clinical use: New implementation patterns”

Robert Van Der Stichele (I~HD) moderated the session “Medicines data integration for clinical use: New implementation patterns“. Today, e-prescribing systems are spreading steadily across Europe. While they are important, these […]
Engaging with pharmacists in Athens

The UNICOM Day in Athens will take place on thursday 18th of January in the context of the HL7 Europe Working Group Meeting and FHIR Marathon. The session is organised […]
IDMP Medicinal products Substitution Workshop: UNICOM day in ATHENS

UNICOM Day – ensure that any medicine and what it contains can be accurately identified anywhere in the world. Improve patient safety and enable better healthcare for all Agenda: 9:30 […]
3rd GIDWG Stakeholder Group Meeting took place in Delft (Holland)
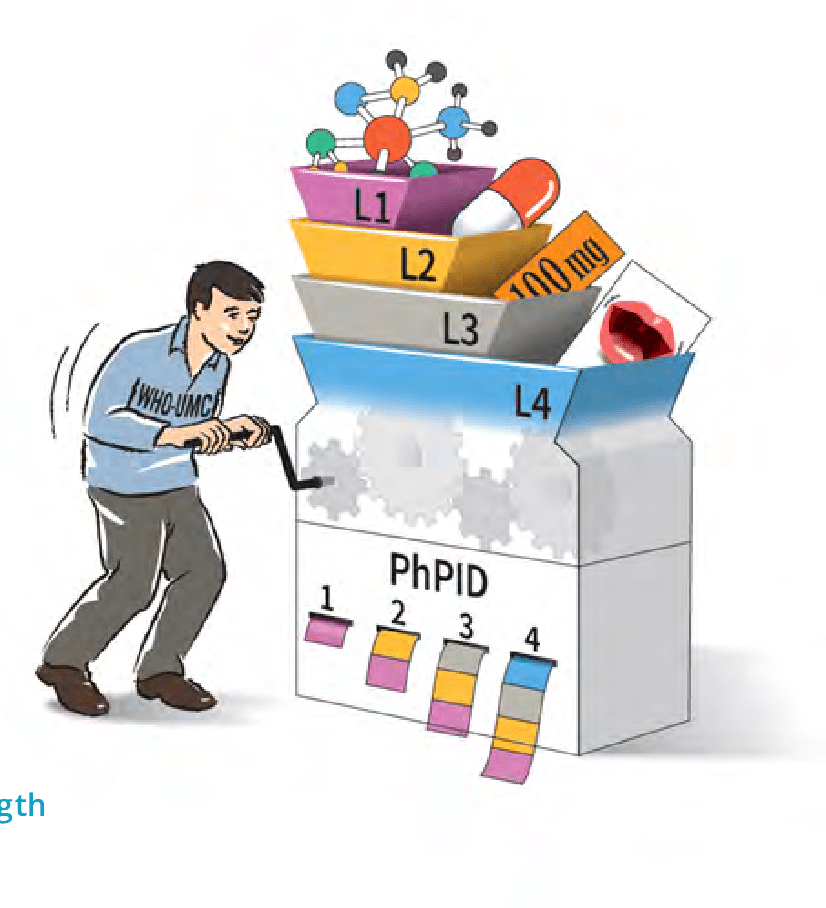
UNICOM is engaged with the Global IDMP Working Group (GIDWG), created by EMA, FDA and WHO-UMC. The 3rd GIDWG Stakeholder Group Meeting took place this week and the results are […]
