FDA refers to UNICOM outputs in its IDMP related webinar

On the 11th of June, the FDA has organised a widely attended webinar on “Identification of Medicinal Products: Path to a Global Implementation”. UNICOM is happy to see that some […]
Join our next Community of Expertise on June 29TH on Cross-border ePrescription
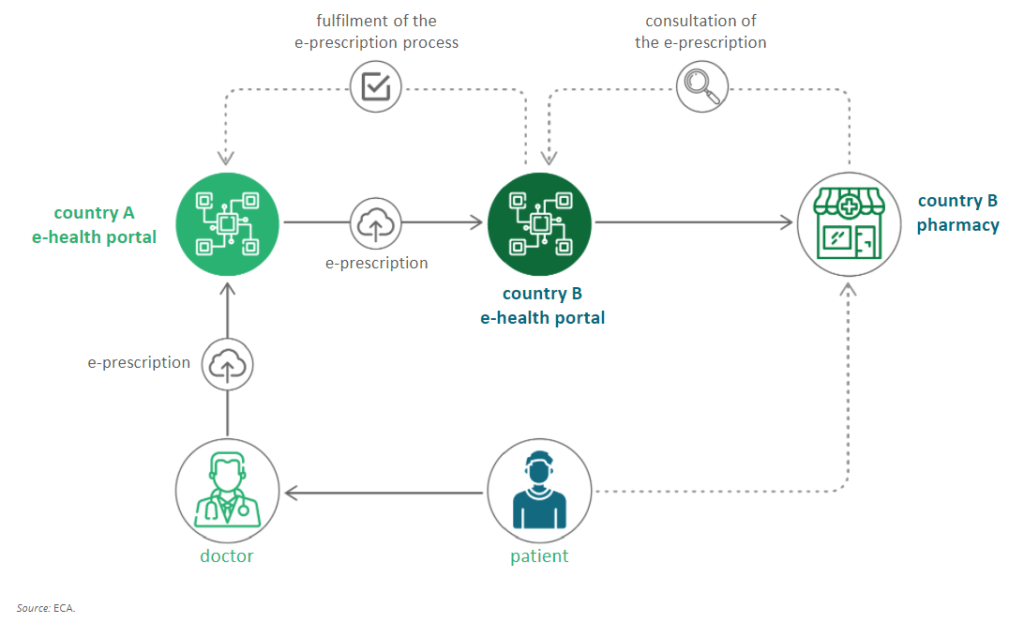
Summer is at our doors and many of us begin to think about travelling abroad. The next UNICOM Community of Expertise is scheduled on Tuesday 29 June 2021 and will thus focus on the very […]
UNICOM raises awareness about the need of IDMP adoption in Greece

As part of its dissemination strategy, UNICOM has decided to focus on a number of EU countries not officially present in the consortium. This is the case of the Greek National […]
IDMP identifiers used for the first time in HL7 FIHR Connecthaton

COVID-19 vaccination in the International Patient Summary using IDMP compliant Identifiers in HL7 FHIR Connectathon 27 On May 17-19, 2021, HL7 organized the 27th FHIR Connectathon (FCAT-27). In the International Patient Summary (FCAT-27 IPS) Track, UNICOM participated exploring the introduction […]
UNICOM now ready to start engaging with North American stakeholders

On the 8th of June, UNICOM will organize its first Transatlantic event. Building on the previous awareness raised by the EU project openMedicine (2016), stakeholders in North America shall learn from UNICOM in an introduction workshop. The workshop organized by […]
Europeans want to be empowered to improve safety and reduce risk when dealing with medicines
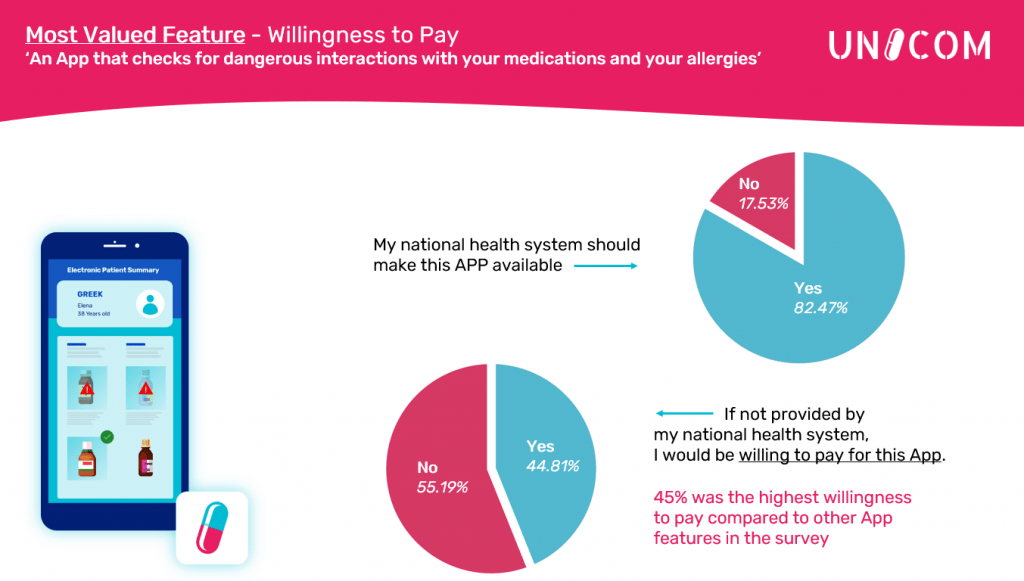
282 people from 27 countries have participated to a public survey organized by UNICOM in February and March 2021 aiming at discovering what is important regarding patient care and empowerment when […]

