Join us on the 28th of May to understand how terminologies such as SNOMED-CT and others need to be aligned to support IDMP related critical use cases.
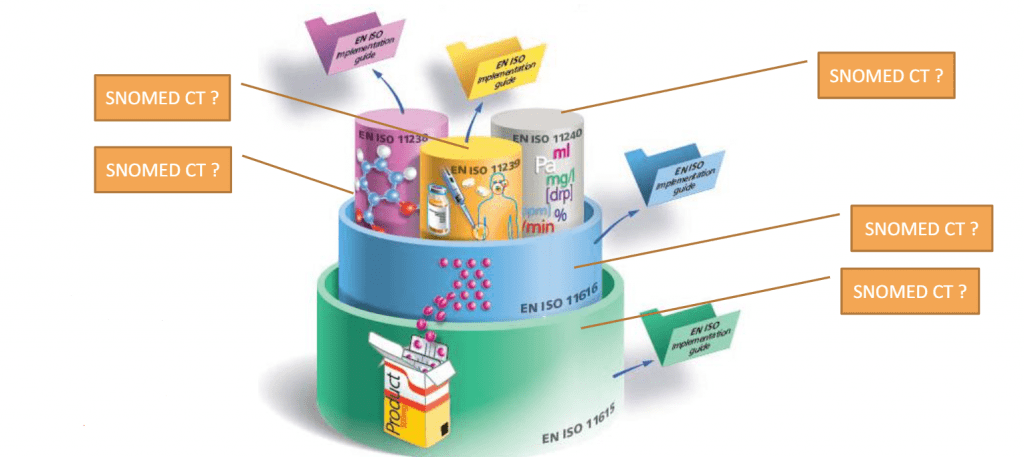
The next UNICOM Community of Expertise is scheduled on Friday 28 May 2021 between 3:00 – 4:30 PM CEST and will focus on the representation of clinical information related to medication using SNOMED CT […]
UNICOM joins the Global Vaccines Initiative and will produce a feasibility assessment on global vaccines substance management by September 2021
While vaccination against Covid-19 is now accelerating in most European countries and worldwide, the question of the correct identification and global alignment on vaccines substances is still for now an open […]
All NCAs of the EU not directly active in the UNICOM project invited now to take part in UNICOM knowledge sharing and best practices webinars.

Thirteen National Competent Authorities (NCAs) for medicinal products are actively involved in the UNICOM project. They are pioneering the implementation of the identification of medicinal products (IDMP) in the European […]
Join the discussion on how ATC and Melclass come together in a medicinal product dictionary at the next UNICOM Community of Expertise scheduled on Thursday 29 April March 2021
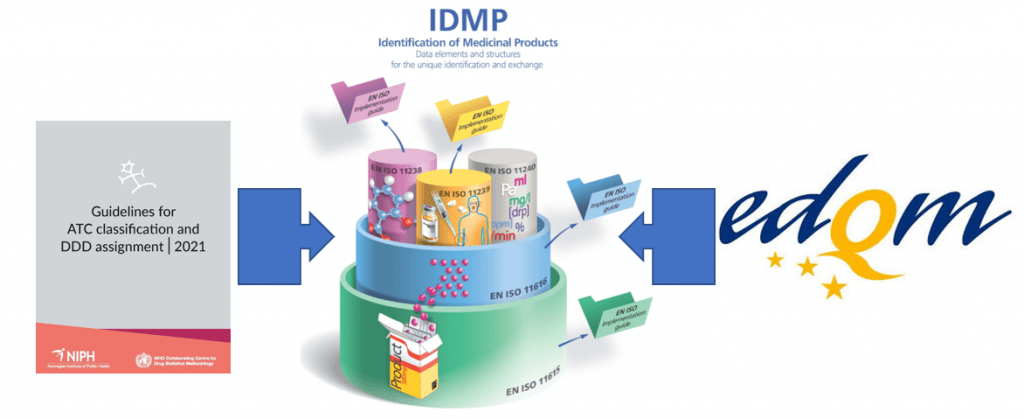
The importance of medicinal product dictionaries (MPDs) is highlighted by two concrete examples. MPDs bring together the information on medicinal products validated by a National Competent Authority and a variety of […]
ISO TC 215, Working Group 6, to commence its standardisation work on an IDMP logical model

On March 11th the rule-based ballot within the ISO TC 215 community for a new work item, has confirmed that the National Mirror Bodies have approved the launch of a project aiming […]
UNICOM supports the further development of the EU cross-border digital health services
Excellent attendance at the 1st UNICOM/eHDSI workshop dedicated to EU eHealth cross-border services More than 80 people attended a two-day workshop organized in February by UNICOM dedicated to the beneficial […]

