UNICOM CARDGAME: Play it!

In the context of the UNICOM testlab, we tried to analyze in more details the data-availability from pharmaceutical companies and the data-needs of regulators, MPD’s and clinicians. The main goal […]
A clinical value chain workshop in Brussels confirms the strategic added-value of IDMP
UNICOM hosted in March 2024 a workshop that aimed to engage stakeholders in discussions about the advantages,challenges, obstacles, and timelines related to the implementation and utilisation of IDMP in the […]
UNICOM and EMA uncover ePI development strategy at RoteListe ePI Summit 2024 in Berlin

This year’s ePI Summit was hosted by ROTE LISTE®. In an era where innovation is transforming every industry, the pharmaceutical sector is no exception. This year’s summit has explored the […]
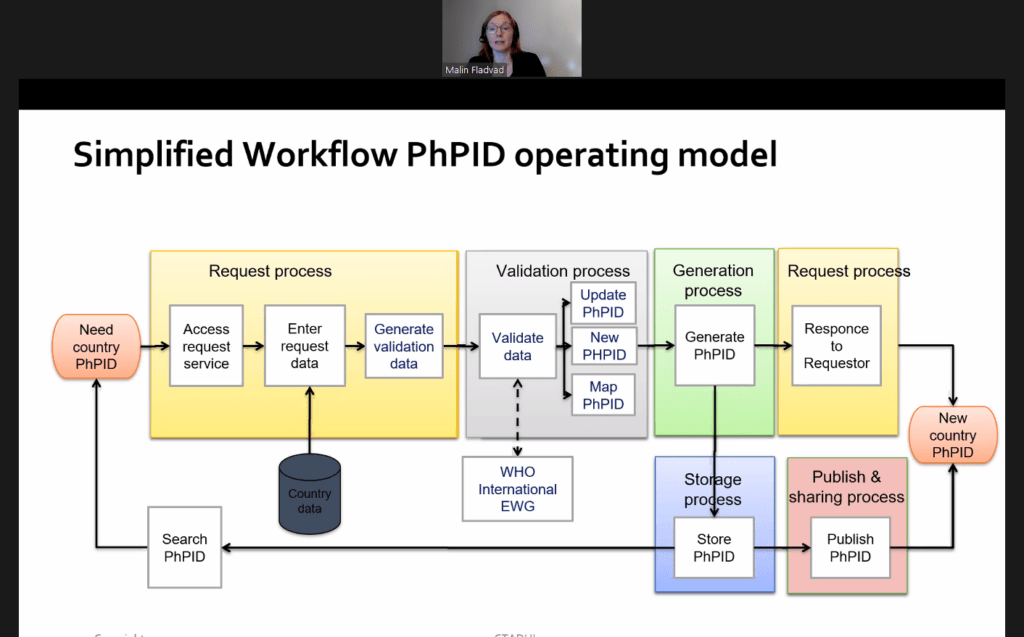
Watch or re-watch UNICOM session: Medicines data integration for clinical use: New implementation patterns”

Robert Van Der Stichele (I~HD) moderated the session “Medicines data integration for clinical use: New implementation patterns“. Today, e-prescribing systems are spreading steadily across Europe. While they are important, these […]
HALMED (Croatia) celebrates 10 years in the EU and welcomes data harmonisation across Europe

In December 2023, It had been 20 years since HALMED had established as a regulatory body for medicinal products and medical devices, and 10 years since HALMED had started taking […]
Engaging with pharmacists in Athens

The UNICOM Day in Athens will take place on thursday 18th of January in the context of the HL7 Europe Working Group Meeting and FHIR Marathon. The session is organised […]

