
NCA KNOWLEDGE TRANSFER: BELGIUM IN FOCUS
VirtualAgenda : 25’ Results of the FAMHP IDMP gap analysis: 1. EMA : deviations (more, less, different) versus ISO IDMP documents (of 2012 and 2017, whichever is more recent) 2. FAMHP […]

Agenda : 25’ Results of the FAMHP IDMP gap analysis: 1. EMA : deviations (more, less, different) versus ISO IDMP documents (of 2012 and 2017, whichever is more recent) 2. FAMHP […]
►You can register for the relevant Webinar by selecting the link below: SPOR and XEVMPD Data Governance - 17 April, 10:00-12:00 CET - https://ema-europa.webex.com/weblink/register/r9ca0c1044ed05282d0fe236251c99a9c Service Desk for SPOR and XEVMPD - 17 […]
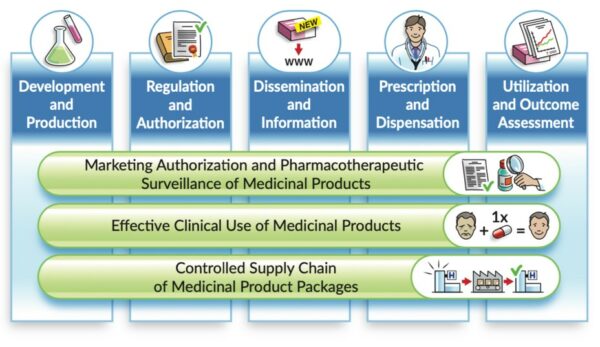
Description: Referring to the landscape of medicinal product data – from “research and development” to “utilisation and outcome assessments”- the vision is that the essential IDMP identifiers remain used and […]
Georg NEUWIRTHER will present UNICOM on May 5th at 10.00 AM. GERMAN SOCIETY FOR REGULATORY AFFAIRS (DGRA) The German Society for Regulatory Affairs e.V. is a scientific specialist society in […]

Central thema: Administrable products : Based on relationship between manufactured and administrable dose form, - NoMA's solution to meet needs in clinical practice. REGISTER

Public webinar intended for business, developers and technical audiences working for industry and national competent authorities that are interested in learning more about the Product Management Service (PMS) development progress and […]
This webinar is targeted at all European NCAs, eHealth competence centers and Industry (EMR, MPDs, HCP and Patient facing Apps). It will present and discuss the approach porposed by IHE […]
UNICOM partner will orchestrate a cross border use case in the interoperability showcase. GNOMON will also make one cross border presentation in the tech leaders stage in the exhibition hall.

You may already REGISTER
Unicom developments around IDMP from a NCA (AGES) perspective by George Neuwirther IDMP Ontology by Heiner Oberkampf and Raphael Sergent EU-SRS by Annet Rozema

A report on progress and challenges from the Global IDMP Working Group (GIDWG), about substances. The presentation will give an overview of the GIDWG work on global alignment of substances, […]

In the context of the IHE-Europe Connectathon WEEK 2023 | Connectathon in Rennes France and in parrallel to the French eHealth week. Looking across the landscape of IDMP implementation, the […]