Intensive preparation and training to prepare the release by EMA of variations form for human medicinal products in october 2022
The Digital Application Dataset Integration (DADI) Network project will replace PDF electronic application forms (eAF) used for regulatory submissions with web-forms, making the future form-filling and submission-handling process more efficient.

UNICOM Day at the IHE Europe Connectathon
Join us in Montreux on September 14 Work Package 1 of the UNICOM project would like to invite you to a dedicated UNICOM day at the IHE Europe Connectathon (CAT)
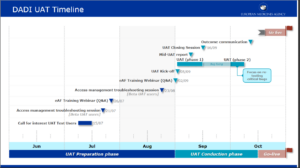
HALMED ( Agency for Medicinal Products and Medical Devices of Croatia) migration journey to IDMP described in an article published today
The authors of the article describe the challenges and learnings in the process of refactoring internal system for tracking the records of marketing authorization procedures and medicinal product database in

Join our next Community of expertise on Friday 26 August 2022: Navigating global and national identifiers using IDMP and FHIR
The next UNICOM Community of Expertise will take place on the 26th of August from 15.00 to 16.30 CEST In collaboration with the Gravitate Health project and the Vulcan Accelerator

A new scientific article published this week in the Journal Applied Science advocates for the use of the EDQM Standard Terms Database for Pharmaceutical Dose Forms
In the context of the UNICOM project, researchers from the European Institute for Innovation through Health Data analysed the Standard Terms for Pharmaceutical Dose Forms from the European Directorate for Quality

Join the UNICOM and GravitateHealth for the next ePi HL7 Vulcan accelerator on May 3 13-14 CEST
During the next HL7 VULCAN Accelerator, the focus will be on the FHIR specification set up by the European Medicines Agency on Electronic Product Information and more partcularly FHIR standard
