NCA Knowledge Transfer Webinar: Croatia implementation progress
Online Event - URL on RegistrationREGISTER NOW FOR THIS EVENT
REGISTER NOW FOR THIS EVENT

Agenda : 25’ Results of the FAMHP IDMP gap analysis: 1. EMA : deviations (more, less, different) versus ISO IDMP documents (of 2012 and 2017, whichever is more recent) 2. FAMHP MPM/DTS : differences with the EMA data model 3. FAMHP approach to narrow the gaps 10’ QUESTIONS 15’ SAM / ePrescription : dataflow from BE […]
►You can register for the relevant Webinar by selecting the link below: SPOR and XEVMPD Data Governance - 17 April, 10:00-12:00 CET - https://ema-europa.webex.com/weblink/register/r9ca0c1044ed05282d0fe236251c99a9c Service Desk for SPOR and XEVMPD - 17 April, 14:00-16:00 CET - https://ema-europa.webex.com/weblink/register/r1552802c0646096c101b5e62b25dfa5d Referentials Management Service (RMS) - 18 April, 10:00-12:00 CET - https://ema-europa.webex.com/weblink/register/r46516995ff51d5f4383d95f05cac8e38 Organisation Management Service (OMS) - 18 April, 14:00-16:00 - https://ema-europa.webex.com/weblink/register/r834ea26426a57529292e0dcffc96cb5a Substance Management […]
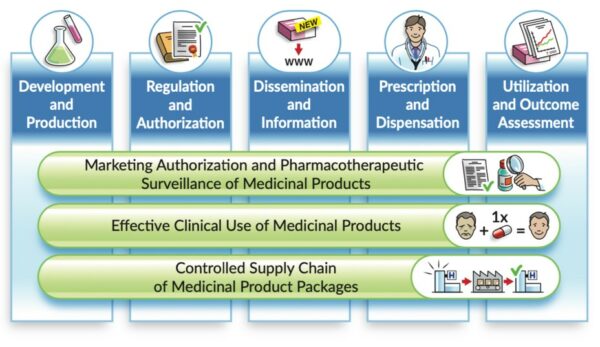
Description: Referring to the landscape of medicinal product data – from “research and development” to “utilisation and outcome assessments”- the vision is that the essential IDMP identifiers remain used and unchanged along that life cycle. This Community of Expertise (CoE) will provide opportunity : for industry to present their perspective and needs, with large international […]

Central thema: Administrable products : Based on relationship between manufactured and administrable dose form, - NoMA's solution to meet needs in clinical practice. REGISTER

Public webinar intended for business, developers and technical audiences working for industry and national competent authorities that are interested in learning more about the Product Management Service (PMS) development progress and related activities. PMS aims to deliver comprehensive and consolidated human medicinal product data (Centrally Authorised Products (CAPs) and Non-CAPs) from different sources. The webinar will be an occasion […]
This webinar is targeted at all European NCAs, eHealth competence centers and Industry (EMR, MPDs, HCP and Patient facing Apps). It will present and discuss the approach porposed by IHE to test and validate IDMP data (using UNICOM resources). The result of this webinar will feed the content of the IHE hacktathon which will take […]

You may already REGISTER

A report on progress and challenges from the Global IDMP Working Group (GIDWG), about substances. The presentation will give an overview of the GIDWG work on global alignment of substances, the role of a global substance ID in the medicinal product lifecycle and the challenges faced. The presentation will also demonstrate how a new resource […]

In the context of the IHE-Europe Connectathon WEEK 2023 | Connectathon in Rennes France and in parrallel to the French eHealth week. Looking across the landscape of IDMP implementation, the UNICOM project aims to develop the following testing scenarios as part of the UNICOM Test Lab: • Submission of variations When a pharmaceutical company needs […]

REGISTER NOW FOR THIS EVENT Advanced FHIR training: How to track changes on a medicinal product? UNICOM is happy to invite all of you to the 4th NCA FHIR training on October 13 10-12 CEST. This webinar focus topic is: “How to track changes on a medicinal product?” "Provenance" in the Variation FHIR message: Free text […]

Agenda: Introduction to Fimea Medicinal product database demo Lessons learned Priority actions expected from EMA Speakers: Joonas Tuominen (Fimea) Anu Ollikainen (Fimea) Markus Mäkelä (Fimea) Juha Jokimäki (Solita Finland Oy)
Unicom will be presenting all the results of WP2 related to SUBSTANCES

Across the UNICOM project we have been actively developing a number of standards-based resources, including FHIR Implementation Guides, a Product Lifecycle Management portal (with EMA), an IDMP database, a substitution service for cross-border dispensation, and a few patient facing apps with dedicated API’s to access medicinal product information (with Gravitate Health). These resources are meant […]

For National Competent Authorities, IDMP implementation is not an option and is already legally compulsory for pharmacovigilance. Industry has already started to submit data in the IDMP format for CAP products variation forms and this will soon be extended to all other forms. NCAs need thus to : Adapt their IT systems (and all other […]

UNICOM Day - ensure that any medicine and what it contains can be accurately identified anywhere in the world. Improve patient safety and enable better healthcare for all Agenda: 9:30 AM - 9:35 AM: Welcome and Introduction (Alexander Berler - GNOMON) SESSION 1 9:35 - 10:00 AM: The promise of IDMP interoperability: the challenge of […]
The webinar is opened to all the staff of all Medicinal Products Agencies. If you want to be invited, send a mail to 1 Welcome: Georg, Sonja Noel 2: PHAROS - Implementation of ISO-IDMP Concepts: Georg, Sonja 3: PHAROS - Importing eAF data based on the new FHIR format (Variation of CPs): Sonja, Noel, […]
The UNICOM project has been working to improve patient safety and enable better healthcare for all, through the implementation of IDMP related standards and terminologies within a variety of processes and systems across the landscape of medicinal product development, use and evaluation. Our key focus has been on the provision of safe medication data, using […]
UNICOM is hosting a workshop that aims to engage stakeholders in discussions about the advantages, challenges, obstacles, and timelines related to the implementation and utilization of IDMP in the clinical realm. This exclusive, one-day event, accessible by invitation only, is designed to showcase the advancements achieved within the UNICOM project and underscore the ongoing efforts […]

This event is not only about presenting the major results achieved by UNICOM over the last four years. It will first of all aim at anchoring those results in a long term perspective and make sure IDMP is part of any major implementation project related to the identification of medicinal products. SEE THE AGENDA: https://unicom-project.eu/wp-content/uploads/2024/02/UNICOM_FinalConference_Agenda_Invitation.pdf […]
Venue: Onsite: COCIR Office, Brussels Online, link will be sent after Registration Description: Multiple interoperability-focused technical artefacts have been developed during the UNICOM project. These artefacts describe how conformant digital health solutions will participate in standards adherent data exchanges. Several European Ecosystems as EHTEL or COCIR members that provide solutions that target European and/or International […]
The webinar will be organised by UNICOM partner HARVARD MEDICAL SCHOOL and has as key objective to update the American interested audience about UNICOM results and discuss in particular some elements of divergence of major importance between the EU and the US . 🗓️ Wed, 05/22/2024 - 12:00 - 13:30 EST (18:00-19:30 CEST) Register Now! […]